Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading
- PMID: 33089895
- PMCID: PMC7821112
- DOI: 10.1111/nph.16946
Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading
Erratum in
-
Corrigendum.New Phytol. 2023 Jun;238(5):2247-2250. doi: 10.1111/nph.18843. Epub 2023 Apr 1. New Phytol. 2023. PMID: 37002836 Free PMC article. No abstract available.
Abstract
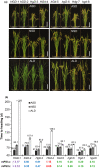
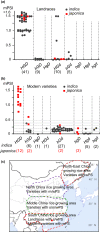
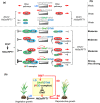
Rice (Oryza sativa) is a short-day (SD) plant originally having strong photoperiod sensitivity (PS), with SDs promoting and long days (LDs) suppressing flowering. Although the evolution of PS in rice has been extensively studied, there are few studies that combine the genetic effects and underlying mechanism of different PS gene combinations with variations in PS. We created a set of isogenic lines among the core PS-flowering genes Hd1, Ghd7 and DTH8 using CRISPR mutagenesis, to systematically dissect their genetic relationships under different day-lengths. We investigated their monogenic, digenic, and trigenic effects on target gene regulation and PS variation. We found that Hd1 and Ghd7 have the primary functions for promoting and repressing flowering, respectively, regardless of day-length. However, under LD conditions, Hd1 promotes Ghd7 expression and is recruited by Ghd7 and/or DTH8 to form repressive complexes that collaboratively suppress the Ehd1-Hd3a/RFT1 pathway to block heading, but under SD conditions Hd1 competes with the complexes to promote Hd3a/RFT1 expression, playing a tradeoff relationship with PS flowering. Natural allelic variations of Hd1, Ghd7 and DTH8 in rice populations have resulted in various PS performances. Our findings reveal that rice PS flowering is controlled by crosstalk of two modules - Hd1-Hd3a/RFT1 in SD conditions and (Hd1/Ghd7/DTH8)-Ehd1-Hd3a/RFT1 in LD conditions - and the divergences of these genes provide the basis for rice adaptation to broad regions.
Keywords: flowering time; heading date; photoperiod sensitivity; rice; short-day plant.
©2020 The Authors New Phytologist ©2020 New Phytologist Foundation.
Figures



Similar articles
-
Hd1, Ghd7, and DTH8 synergistically determine the rice heading date and yield-related agronomic traits.J Genet Genomics. 2022 May;49(5):437-447. doi: 10.1016/j.jgg.2022.02.018. Epub 2022 Mar 4. J Genet Genomics. 2022. PMID: 35248762
-
The DTH8-Hd1 Module Mediates Day-Length-Dependent Regulation of Rice Flowering.Mol Plant. 2017 Jul 5;10(7):948-961. doi: 10.1016/j.molp.2017.05.006. Epub 2017 May 23. Mol Plant. 2017. PMID: 28549969
-
Alternative functions of Hd1 in repressing or promoting heading are determined by Ghd7 status under long-day conditions.Sci Rep. 2017 Jul 14;7(1):5388. doi: 10.1038/s41598-017-05873-1. Sci Rep. 2017. PMID: 28710485 Free PMC article.
-
Transcriptional and post-transcriptional regulation of heading date in rice.New Phytol. 2021 May;230(3):943-956. doi: 10.1111/nph.17158. Epub 2021 Feb 14. New Phytol. 2021. PMID: 33341945 Free PMC article. Review.
-
Research progress of photoperiod regulated genes on flowering time in rice.Yi Chuan. 2016 Jun 20;38(6):532-542. doi: 10.16288/j.yczz.15-478. Yi Chuan. 2016. PMID: 27655315 Review.
Cited by
-
Genome-Wide Analysis of CCT Transcript Factors to Identify Genes Contributing to Photoperiodic Flowering in Oryza rufipogon.Front Plant Sci. 2021 Nov 8;12:736419. doi: 10.3389/fpls.2021.736419. eCollection 2021. Front Plant Sci. 2021. PMID: 34819938 Free PMC article.
-
The phosphoinositide-specific phospholipase C1 modulates flowering time and grain size in rice.Planta. 2022 Jul 4;256(2):29. doi: 10.1007/s00425-022-03941-z. Planta. 2022. PMID: 35781561
-
Oryza CLIMtools: A Genome-Environment Association Resource Reveals Adaptive Roles for Heterotrimeric G Proteins in the Regulation of Rice Agronomic Traits.bioRxiv [Preprint]. 2023 Dec 21:2023.05.10.540241. doi: 10.1101/2023.05.10.540241. bioRxiv. 2023. Update in: Plant Commun. 2024 Apr 8;5(4):100813. doi: 10.1016/j.xplc.2024.100813. PMID: 37214799 Free PMC article. Updated. Preprint.
-
Is the time of anthesis in rice (Oryza sativa) influenced by photoperiod?Biol Futur. 2024 Dec;75(4):453-458. doi: 10.1007/s42977-024-00223-5. Epub 2024 May 14. Biol Futur. 2024. PMID: 38744795
-
Adaptive response of cultivated rice (Oryza sativa ssp. indica) flowers to low sunlight intensity.Biol Futur. 2025 Aug 12. doi: 10.1007/s42977-025-00277-z. Online ahead of print. Biol Futur. 2025. PMID: 40797148
References
-
- Bian XF, Liu X, Zhao ZG, Jiang L, Gao H, Zhang YH, Zheng M, Chen LM, Liu SJ, Zhai HQ et al 2011. Heading date gene, dth3 controlled late flowering in O. Glaberrima Steud. by down‐regulating Ehd1 . Plant Cell Reports 30: 2243–2254. - PubMed
-
- Brambilla V, Fornara F. 2013. Molecular control of flowering in response to day length in rice. Journal of Integrative Plant Biology 55: 410–418. - PubMed
-
- Brambilla V, Fornara F. 2017. Y flowering? Regulation and activity of CONSTANS and CCT‐domain proteins in Arabidopsis and crop species. Biochimica et Biophysica Acta (BBA) ‐ Gene Regulatory Mechanisms 1860: 655–660. - PubMed
-
- Cai MH, Chen SH, Wu MM, Zheng TH, Zhou L, Li CN, Zhang H, Wang JC, Xu XY, Chai JT et al 2019. Early heading 7 interacts with DTH8, and regulates flowering time in rice. Plant Cell Reports 38: 521–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

