Fast Acting Insulin Aspart Compared with Insulin Aspart in the Medtronic 670G Hybrid Closed Loop System in Type 1 Diabetes: An Open Label Crossover Study
- PMID: 33090016
- PMCID: PMC7994433
- DOI: 10.1089/dia.2020.0500
Fast Acting Insulin Aspart Compared with Insulin Aspart in the Medtronic 670G Hybrid Closed Loop System in Type 1 Diabetes: An Open Label Crossover Study
Abstract
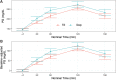
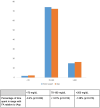
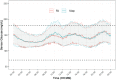
This is a single-center randomized open label active-controlled crossover trial comparing efficacy and safety of fast acting insulin aspart (FA) (FIASP®) versus insulin aspart (IAsp) (NovoLog®) when used in the Medtronic 670G system in auto mode in patients with type 1 diabetes. Forty patients were randomized to either IAsp or FA. Each treatment period was 7 weeks and a standardized meal test was administered 6 weeks after the start of each treatment period. The primary endpoint was postprandial glucose (PPG) increment after the meal test at 1 h. Treatment with FA using the MiniMed 670G hybrid closed loop (HCL) led to a greater reduction in 1-h postprandial glucose increase compared with treatment with IAsp during the standardized mixed meal test. Change in glucose: [estimated treatment difference (ETD ± standard deviation [SD]); 95% confidence interval]: 70.27 (±17.36) mg/dL (3.9 ± 1.0 mmol/L) with FA versus 98.42 (±17.36) mg/dL (5.5 ± 1.0 mmol/L) with IAsp (P = 0.008). Patients spent 1.81% (P = 0.016) more time (equivalent to 26 min per day) in the 70-180 mg/dL (3.89-9.99 mmol/L) range with FA than with IAsp. The entire sample spent only 0.5% of time <54 mg/dL (<3.0 mmol/L) range. The increment in the 1 h postmeal test glucose was significantly lower with FA versus IAsp. FA in a HCL setting is safe and effective with patients spending more time in the 70-180 mg/dL (3.89-9.99 mmol/L) target range than with IAsp. Trial registration: Clinicaltrials.gov identifier: NCT03977727.
Keywords: Fast acting insulin aspart; Medtronic 670G hybrid closed loop; Type 1 diabetes.
Conflict of interest statement
K.O. reports clinical research support from Lilly, Allergan, Sanofi, Abbott, Medtronic, Senseonics, and Dexcom and is on the speaker's bureau for Novo Nordisk, Eli Lilly, BI, AZ, Janssen, Clarus Therapeutics, IBSA. He has served on advisory boards for Eli Lilly and IBSA. T.B. reports clinical research support from Novo Nordisk, Lilly, Allergan, Senseonics, Eli Lilly, Sanofi, BI, AZ, Janssen, Amgen, Dexcom, and Abbott. He has served on advisory boards for Intarcia and Eli Lilly. A.C. reports being on clinical trial retention panels for Novo Nordisk and performing consulting work for Eli Lilly.
Figures

References
-
- Bergenstal RM, Garg SK, Weinzimer SA: Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 - PubMed
-
- Russell-Jones D, Bode B, De Block C, et al. : Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: Results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (onset 1). Diabetes Care 2017;40:943–950 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
