Impact of Immune Priming, Vaccination, and Infection on Influenza A(H3N2) Antibody Landscapes in Children
- PMID: 33090202
- PMCID: PMC8145779
- DOI: 10.1093/infdis/jiaa665
Impact of Immune Priming, Vaccination, and Infection on Influenza A(H3N2) Antibody Landscapes in Children
Abstract
Background: Preexisting antibodies to influenza, shaped by early infection and subsequent exposures, may impact responses to influenza vaccination.
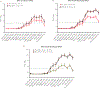
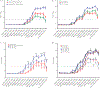
Methods: We enrolled 72 children (aged 7-17 years) in 2015-2016; all received inactivated influenza vaccines. Forty-one were also vaccinated in 2014-2015, with 12 becoming infected with A(H3N2) in 2014-2015. Thirty-one children did not have documented influenza exposures in the prior 5 seasons. Sera were collected pre- and postvaccination in both seasons. We constructed antibody landscapes using hemagglutination inhibition antibody titers against 16 A(H3N2) viruses representative of major antigenic clusters that circulated between 1968 and 2015.
Results: The breadth of the antibody landscapes increased with age. Vaccine-induced antibody responses correlated with boosting of titers to previously encountered antigens. Postvaccination titers were the highest against vaccine antigens rather than the historic A(H3N2) viruses previously encountered. Prevaccination titers to the vaccine were the strongest predictors of postvaccination titers. Responses to vaccine antigens did not differ by likely priming virus. Influenza A(H3N2)-infected children in 2014-2015 had narrower antibody landscapes than those uninfected, but prior season infection status had little effect on antibody landscapes following 2015-2016 vaccination.
Conclusions: A(H3N2) antibody landscapes in children were largely determined by age-related immune priming, rather than recent vaccination or infection.
Keywords: antibody landscape; birth cohorts; immune priming; infection; influenza vaccination.
Published by Oxford University Press for the Infectious Diseases Society of America 2020.
Conflict of interest statement
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures




References
-
- Henkle E, Irving SA, Naleway AL, et al. Comparison of laboratory-confirmed influenza and noninfluenza acute respiratory illness in healthcare personnel during the 2010–2011 influenza season. Infect Control Hosp Epidemiol 2014; 35:538–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

