Parallels between retinal and brain pathology and response to immunotherapy in old, late-stage Alzheimer's disease mouse models
- PMID: 33090673
- PMCID: PMC7681044
- DOI: 10.1111/acel.13246
Parallels between retinal and brain pathology and response to immunotherapy in old, late-stage Alzheimer's disease mouse models
Abstract
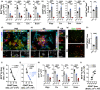
Despite growing evidence for the characteristic signs of Alzheimer's disease (AD) in the neurosensory retina, our understanding of retina-brain relationships, especially at advanced disease stages and in response to therapy, is lacking. In transgenic models of AD (APPSWE/PS1∆E9; ADtg mice), glatiramer acetate (GA) immunomodulation alleviates disease progression in pre- and early-symptomatic disease stages. Here, we explored the link between retinal and cerebral AD-related biomarkers, including response to GA immunization, in cohorts of old, late-stage ADtg mice. This aged model is considered more clinically relevant to the age-dependent disease. Levels of synaptotoxic amyloid β-protein (Aβ)1-42, angiopathic Aβ1-40, non-amyloidogenic Aβ1-38, and Aβ42/Aβ40 ratios tightly correlated between paired retinas derived from oculus sinister (OS) and oculus dexter (OD) eyes, and between left and right posterior brain hemispheres. We identified lateralization of Aβ burden, with one-side dominance within paired retinal and brain tissues. Importantly, OS and OD retinal Aβ levels correlated with their cerebral counterparts, with stronger contralateral correlations and following GA immunization. Moreover, immunomodulation in old ADtg mice brought about reductions in cerebral vascular and parenchymal Aβ deposits, especially of large, dense-core plaques, and alleviation of microgliosis and astrocytosis. Immunization further enhanced cerebral recruitment of peripheral myeloid cells and synaptic preservation. Mass spectrometry analysis identified new parallels in retino-cerebral AD-related pathology and response to GA immunization, including restoration of homeostatic glutamine synthetase expression. Overall, our results illustrate the viability of immunomodulation-guided CNS repair in old AD model mice, while shedding light onto similar retino-cerebral responses to intervention, providing incentives to explore retinal AD biomarkers.
Keywords: astrocytes reactivation; glutamine synthetase; myeloid cells; neurodegenerative disease; ocular proteins; retina; synaptic preservation; vascular amyloidosis.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
Y.K., M.K.H., and K.L.B. are co‐founders and stockholders of NeuroVision Imaging, Inc., 1395 Garden Highway, Suite 250, Sacramento, CA 95833, USA.
Figures




References
-
- Alzheimer's Association . (2018). 2018 Alzheimer's disease facts and figures. Alzheimer's & Dementia, 14(3), 367–429. 10.1016/j.jalz.2018.02.001 - DOI
-
- Asanad, S. , Ross‐Cisneros, F. N. , Barron, E. , Nassisi, M. , Sultan, W. , Karanjia, R. , & Sadun, A. A. (2019). The retinal choroid as an oculovascular biomarker for Alzheimer's dementia: A histopathological study in severe disease. Alzheimer's & Dementia: the Journal of the Alzheimer's Association, 11, 775–783. 10.1016/j.dadm.2019.08.005 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

