High levels of modified ceramides are a defining feature of murine and human cancer cachexia
- PMID: 33090732
- PMCID: PMC7749558
- DOI: 10.1002/jcsm.12626
High levels of modified ceramides are a defining feature of murine and human cancer cachexia
Abstract
Background: Cancer cachexia (CCx) is a multifactorial energy-wasting syndrome reducing the efficiency of anti-cancer therapies, quality of life, and survival of cancer patients. In the past years, most studies focused on the identification of tumour and host-derived proteins contributing to CCx. However, there is still a lack of studies addressing the changes in bioactive lipids. The aim of this study was to identify specific lipid species as a hallmark of CCx by performing a broad range lipid analysis of plasma from well-established CCx mouse models as well as cachectic and weight stable cancer patients.
Methods: Plasma from non-cachectic (PBS-injected mice, NC26 tumour-bearing mice), pre-cachectic and cachectic mice (C26 and LLC tumour-bearing mice, ApcMin/+ mutant mice), and plasma from weight stable and cachectic patients with gastrointestinal cancer, were analysed using the Lipidyzer™ platform. In total, 13 lipid classes and more than 1100 lipid species, including sphingolipids, neutral and polar glycerolipids, were covered by the analysis. Correlation analysis between specific lipid species and readouts of CCx were performed. Lipidomics data were confirmed by gene expression analysis of metabolic organs to analyse enzymes involved in sphingolipid synthesis and degradation.
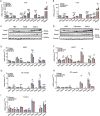
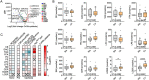
Results: A decrease in several lysophosphatidylcholine (LPC) species and an increase in numerous sphingolipids including sphingomyelins (SMs), ceramides (CERs), hexosyl-ceramides (HCERs) and lactosyl-ceramides (LCERs), were mutual features of CCx in both mice and cancer patients. Notably, sphingolipid levels gradually increased during cachexia development. Key enzymes involved in ceramide synthesis were elevated in liver but not in adipose, muscle, or tumour tissues, suggesting that ceramide turnover in the liver is a major contributor to elevated sphingolipid levels in CCx. LPC(16:1), LPC(20:3), SM(16:0), SM(24:1), CER(16:0), CER(24:1), HCER(16:0), and HCER(24:1) were the most consistently affected lipid species between mice and humans and correlated negatively (LPCs) or positively (SMs, CERs and HCERs) with the severity of body weight loss.
Conclusions: High levels of sphingolipids, specifically ceramides and modified ceramides, are a defining feature of murine and human CCx and may contribute to tissue wasting and skeletal muscle atrophy through the inhibition of anabolic signals. The progressive increase in sphingolipids during cachexia development supports their potential as early biomarkers for CCx.
Keywords: Cancer cachexia; Ceramides; Lipidomics; Signalling lipids; Sphingolipids.
© 2020 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures



Comment in
-
Biomarkers for cancer cachexia: where do we stand?J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1388-1389. doi: 10.1002/jcsm.12641. Epub 2020 Nov 30. J Cachexia Sarcopenia Muscle. 2020. PMID: 33258306 Free PMC article. No abstract available.
References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
-
- Argiles JM, Stemmler B, López‐Soriano FJ, Busquets S. Inter‐tissue communication in cancer cachexia. Nat Rev Endocrinol 2018;15:9–20. - PubMed
-
- Schmidt SF, Rohm M, Herzig S, Diaz MB. Cancer cachexia: more than skeletal muscle wasting. Trends Cancer 2018;4:849–860. - PubMed
-
- Argiles JM, López‐Soriano FJ, Stemmler B, Busquets S. Novel targeted therapies for cancer cachexia. Biochem J 2017;474:2663–2678. - PubMed
-
- Biswas AK, Acharyya S. Understanding cachexia in the context of metastatic progression. Nat Rev Cancer 2020;20:274–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

