Clinical Endpoints for Evaluating Efficacy in COVID-19 Vaccine Trials
- PMID: 33090877
- PMCID: PMC7596738
- DOI: 10.7326/M20-6169
Clinical Endpoints for Evaluating Efficacy in COVID-19 Vaccine Trials
Abstract
Several vaccine candidates to protect against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or coronavirus disease 2019 (COVID-19) have entered or will soon enter large-scale, phase 3, placebo-controlled randomized clinical trials. To facilitate harmonized evaluation and comparison of the efficacy of these vaccines, a general set of clinical endpoints is proposed, along with considerations to guide the selection of the primary endpoints on the basis of clinical and statistical reasoning. The plausibility that vaccine protection against symptomatic COVID-19 could be accompanied by a shift toward more SARS-CoV-2 infections that are asymptomatic is highlighted, as well as the potential implications of such a shift.
Conflict of interest statement
Figures
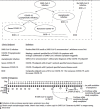

References
-
- Cohen MS, Corey L. Combination prevention for COVID-19 [Editorial]. Science. 2020;368:551. [PMID: 32381692] doi:10.1126/science.abc5798 - PubMed
-
- Corey L, Mascola JR, Fauci AS, et al. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368:948-950. [PMID: 32393526] doi:10.1126/science.abc5312 - PubMed
-
- World Health Organization. An international randomised trial of candidate vaccines against COVID-19. Accessed at www.who.int/publications-detail/an-international-randomised-trial-of-can... on 26 July 2020.
-
- World Health Organization. Draft landscape of COVID-19 candidate vaccines. Accessed at www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-va... on 2 August 2020.
-
- U.S. Department of Health and Human Services, U.S. Food and Drug Administration, Center for Biologics Evaluation and Research. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry. Accessed at www.fda.gov/media/139638/download on 24 July 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
