Gallbladder cancer revisited: the evolving role of a radiologist
- PMID: 33090880
- PMCID: PMC7774702
- DOI: 10.1259/bjr.20200726
Gallbladder cancer revisited: the evolving role of a radiologist
Abstract
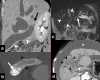
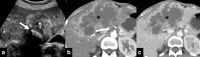
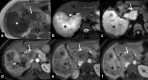
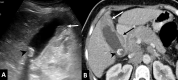
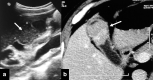
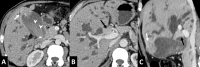
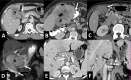
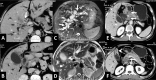
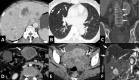
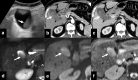
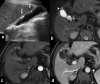
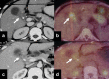
Gallbladder cancer is the most common malignancy of the biliary tract. It is also the most aggressive biliary tumor with the shortest median survival duration. Complete surgical resection, the only potentially curative treatment, can be accomplished only in those patients who are diagnosed at an early stage of the disease. Majority (90%) of the patients present at an advanced stage and the management involves a multidisciplinary approach. The role of imaging in gallbladder cancer cannot be overemphasized. Imaging is crucial not only in detecting, staging, and planning management but also in guiding radiological interventions. This article discusses the role of a radiologist in the diagnosis and management of gallbladder cancer.
Figures






References
-
- Ertel AE, Bentrem D, Abbott DE, Cancer GB. Gall bladder cancer : Bentrem D, Benson AB, Gastrointestinal malignancies. Cham: Springer International Publishing; 2016. 101–20.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

