Candida auris: Epidemiology, biology, antifungal resistance, and virulence
- PMID: 33091071
- PMCID: PMC7581363
- DOI: 10.1371/journal.ppat.1008921
Candida auris: Epidemiology, biology, antifungal resistance, and virulence
Abstract
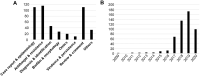
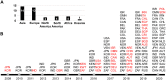
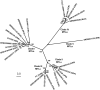
First described in 2009 in Japan, the emerging multidrug-resistant fungal pathogen Candida auris is becoming a worldwide public health threat that has been attracting considerable attention due to its rapid and widespread emergence over the past decade. The reasons behind the recent emergence of this fungus remain a mystery to date. Genetic analyses indicate that this fungal pathogen emerged simultaneously in several different continents, where 5 genetically distinct clades of C. auris were isolated from distinct geographical locations. Although C. auris belongs to the CTG clade (its constituent species translate the CTG codon as serine instead of leucine, as in the standard code), C. auris is a haploid fungal species that is more closely related to the haploid and often multidrug-resistant species Candida haemulonii and Candida lusitaniae and is distantly related to the diploid and clinically common fungal pathogens Candida albicans and Candida tropicalis. Infections and outbreaks caused by C. auris in hospitals settings have been rising over the past several years. Difficulty in its identification, multidrug resistance properties, evolution of virulence factors, associated high mortality rates in patients, and long-term survival on surfaces in the environment make C. auris particularly problematic in clinical settings. Here, we review progress made over the past decade on the biological and clinical aspects of C. auris. Future efforts should be directed toward understanding the mechanistic details of its biology, epidemiology, antifungal resistance, and pathogenesis with a goal of developing novel tools and methods for the prevention, diagnosis, and treatment of C. auris infections.
Conflict of interest statement
Clarissa J. Nobile is a cofounder of BioSynesis, Inc., a company developing inhibitors and diagnostics of biofilms.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

