α-Synuclein modulates tau spreading in mouse brains
- PMID: 33091110
- PMCID: PMC7588140
- DOI: 10.1084/jem.20192193
α-Synuclein modulates tau spreading in mouse brains
Abstract
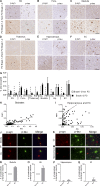
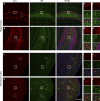
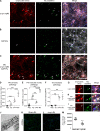
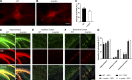
α-Synuclein (α-syn) and tau aggregates are the neuropathological hallmarks of Parkinson's disease (PD) and Alzheimer's disease (AD), respectively, although both pathologies co-occur in patients with these diseases, suggesting possible crosstalk between them. To elucidate the interactions of pathological α-syn and tau, we sought to model these interactions. We show that increased accumulation of tau aggregates occur following simultaneous introduction of α-syn mousepreformed fibrils (mpffs) and AD lysate-derived tau seeds (AD-tau) both in vitro and in vivo. Interestingly, the absence of endogenous mouse α-syn in mice reduces the accumulation and spreading of tau, while the absence of tau did not affect the seeding or spreading capacity of α-syn. These in vivo results are consistent with our in vitro data wherein the presence of tau has no synergistic effects on α-syn. Our results point to the important role of α-syn as a modulator of tau pathology burden and spreading in the brains of AD, PDD, and DLB patients.
© 2020 Bassil et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







References
-
- Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W.H., Castillo P.E., Shinsky N., Verdugo J.M., Armanini M., Ryan A., et al. . 2000. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 25:239–252. 10.1016/S0896-6273(00)80886-7 - DOI - PubMed
-
- Badiola N., de Oliveira R.M., Herrera F., Guardia-Laguarta C., Gonçalves S.A., Pera M., Suárez-Calvet M., Clarimon J., Outeiro T.F., and Lleó A.. 2011. Tau enhances α-synuclein aggregation and toxicity in cellular models of synucleinopathy. PLoS One. 6:e26609 10.1371/journal.pone.0026609 - DOI - PMC - PubMed
-
- Bassil F., Brown H.J., Pattabhiraman S., Iwasyk J.E., Maghames C.M., Meymand E.S., Cox T.O., Riddle D.M., Zhang B., Trojanowski J.Q., and Lee V.M.. 2020. Amyloid-Beta (Aβ) Plaques Promote Seeding and Spreading of Alpha-Synuclein and Tau in a Mouse Model of Lewy Body Disorders with Aβ Pathology. Neuron. 105:260–275.e6. 10.1016/j.neuron.2019.10.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

