Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review
- PMID: 33092058
- PMCID: PMC7589568
- DOI: 10.3390/antibiotics9100716
Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review
Abstract
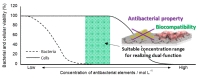
Titanium (Ti) and its alloys are commonly used in medical devices. However, biomaterial-associated infections such as peri-implantitis and prosthetic joint infections are devastating and threatening complications for patients, dentists, and orthopedists and are easily developed on titanium surfaces. Therefore, this review focuses on the formation of biofilms on implant surfaces, which is the main cause of infections, and one-step micro-arc oxidation (MAO) as a coating technology that can be expected to prevent infections due to the implant. Many researchers have provided sufficient data to prove the efficacy of MAO for preventing the initial stages of biofilm formation on implant surfaces. Silver (Ag), copper (Cu), and zinc (Zn) are well used and are incorporated into the Ti surface by MAO. In this review, the antibacterial properties, cytotoxicity, and durability of these elements on the Ti surface incorporated by one-step MAO will be summarized. This review is aimed at enhancing the importance of the quantitative control of Ag, Cu, and Zn for their use in implant surfaces and the significance of the biodegradation behavior of these elements for the development of antibacterial properties.
Keywords: antibacterial properties; biofilm; coating; copper; implant; infection; micro-arc oxidation; silver; titanium; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Brånemark P.I., Hansson B.O., Adell R., Breine U., Lindström J., Hallén O., Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstruct. Surg. Hand Surg. 1977;11(Suppl. S16):1–132. - PubMed
-
- Listgarten M.A., Buser D., Steinemann S.G., Donath K., Lang N.P., Weber H.P. Light and transmission electron microscopy of the intact interfaces between non-submerged titanium-coated epoxy resin implants and bone or gingiva. J. Dent. Res. 1992;71:364–371. doi: 10.1177/00220345920710020401. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

