Patient-Derived Xenograft vs. Organoids: A Preliminary Analysis of Cancer Research Output, Funding and Human Health Impact in 2014-2019
- PMID: 33092060
- PMCID: PMC7593921
- DOI: 10.3390/ani10101923
Patient-Derived Xenograft vs. Organoids: A Preliminary Analysis of Cancer Research Output, Funding and Human Health Impact in 2014-2019
Abstract
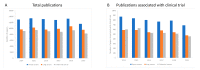
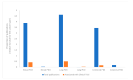
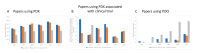
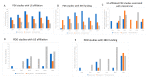
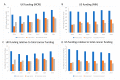
Cancer remains a major threat to mortality and morbidity globally, despite intense research and generous funding. Patient-derived xenograft (PDX) models-where tumor biopsies are injected into an animal-were developed to improve the predictive capacity of preclinical animal models. However, recent observations have called into question the clinical relevance, and therefore the translational accuracy, of these. Patient-derived organoids (PDO) use patient tumor samples to create in vitro models that maintain aspects of tumor structure and heterogeneity. We undertook a preliminary analysis of the number of breast, colorectal, and lung cancer research studies using PDX or PDO published worldwide between 2014-2019. We looked for evidence of impacts of this research on human health. The number of publications that focused on PDO is gradually increasing over time, but is still very low compared to publications using PDX models. Support for new research projects using PDO is gradually increasing, a promising indicator of a shift towards more human-relevant approaches to understanding human disease. Overall, increases in total funding for these three major cancer types does not appear to be translating to any consequential increase in outputs, defined for this purpose as publications associated with clinical trials. With increasing public discomfort in research using animals and demands for 'alternative' methods, it is timely to consider how to implement non-animal methods more effectively.
Keywords: breast cancer; colorectal cancer; lung cancer; patient-derived organoids; patient-derived xenografts; research outputs.
Conflict of interest statement
The authors all work for Humane Society International, an animal protection organization that aims to promote the use of non-animal methodologies in research and testing, with a view to the ultimate replacement of animals in science.
Figures
References
-
- Globocan All Cancers Fact Sheet; 2018 International Agency for Research on Cancer. [(accessed on 27 February 2020)]; Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sh....
LinkOut - more resources
Full Text Sources

