Frequency and Duration of SARS-CoV-2 Shedding in Oral Fluid Samples Assessed by a Modified Commercial Rapid Molecular Assay
- PMID: 33092065
- PMCID: PMC7589602
- DOI: 10.3390/v12101184
Frequency and Duration of SARS-CoV-2 Shedding in Oral Fluid Samples Assessed by a Modified Commercial Rapid Molecular Assay
Abstract
Background: RT-PCR on nasopharyngeal (NPS)/oropharyngeal swabs is the gold standard for diagnosis of SARS-CoV-2 infection and viral load monitoring. Oral fluid (OF) is an alternate clinical sample, easy and safer to collect and could be useful for COVID-19 diagnosis, monitoring viral load and shedding.
Methods: Optimal assay conditions and analytical sensitivity were established for the commercial Simplexa™ COVID-19 Direct assay adapted to OF matrix. The assay was used to test 337 OF and NPS specimens collected in parallel from 164 hospitalized patients; 50 bronchoalveolar lavage (BAL) specimens from a subgroup of severe COVID-19 cases were also analysed.
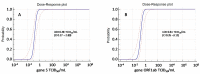
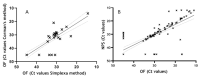
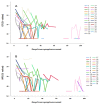
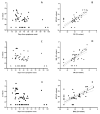
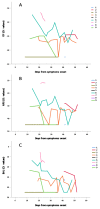
Results: Using Simplexa™ COVID-19 Direct on OF matrix, 100% analytical detection down to 1 TCID50/mL (corresponding to 4 × 103 copies (cp)/mL) was observed. No crossreaction with other viruses transmitted through the respiratory toute was observed. Parallel testing of 337 OF and NPS samples showed highly concordant results (κ = 0.831; 95 % CI = 0.771-0.891), and high correlation of Ct values (r = 0.921; p < 0.0001). High concordance and elevated correlation was observed also between OF and BAL. Prolonged viral RNA shedding was observed up to 100 days from symptoms onset (DSO), with 32% and 29% positivity observed in OF and NPS samples, respectively, collected between 60 and 100 DSO.
Conclusions: Simplexa™ COVID-19 Direct assays on OF have high sensitivity and specificity to detect SARS-CoV-2 RNA and provide an alternative to NPS for diagnosis and monitoring SARS-CoV-2 shedding.
Keywords: SARS-CoV-2 RNA; direct assay; oral fluid; real time RT-PCR.
Conflict of interest statement
Giulia Minnucci and Elena D’Agostino are employees of the DiaSorin S.p.A. (Gerenzano, Italy). In no way their contribution to the completion of the study influenced the study design and the analysis of the results. No additional conflict of interest or other competing relationships exist.
Figures

References
-
- The World Health Organization Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases. Interim guidance 2 March 2020. [(accessed on 18 March 2020)]; Available online: https://www.who.int/publications-detail/laboratory-testing-for-2019-nove....
-
- ECDC Novel Coronavirus (SARS-CoV-2) Discharge Criteria for Confirmed COVID-19 Cases e when Is It Safe to Discharge COVID-19 Cases from the Hospital or end Home Isolation? [(accessed on 29 March 2020)]; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-Discha....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

