Evaluation of Cytotoxicity and α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids
- PMID: 33092246
- PMCID: PMC7587962
- DOI: 10.3390/molecules25204833
Evaluation of Cytotoxicity and α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids
Abstract
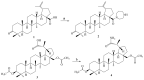
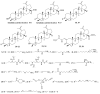
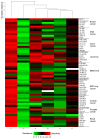
A series of two new and twenty earlier synthesized branched extra-amino-triterpenoids obtained by the direct coupling of betulinic/betulonic acids with polymethylenpolyamines, or by the cyanoethylation of lupane type alcohols, oximes, amines, and amides with the following reduction were evaluated for cytotoxicity toward the NCI-60 cancer cell line panel, α-glucosidase inhibitory, and antimicrobial activities. Lupane carboxamides, conjugates with diaminopropane, triethylenetetramine, and branched C3-cyanoethylated polyamine methyl betulonate showed high cytotoxic activity against most of the tested cancer cell lines with GI50 that ranged from 1.09 to 54.40 µM. Betulonic acid C28-conjugate with triethylenetetramine and C3,C28-bis-aminopropoxy-betulin were found to be potent micromolar inhibitors of yeast α-glucosidase and to simultaneously inhibit the endosomal reticulum α-glucosidase, rendering them as potentially capable to suppress tumor invasiveness and neovascularization, in addition to the direct cytotoxicity. Plausible mechanisms of cytotoxic action and underlying disrupted molecular pathways were elucidated with CellMinner pattern analysis and Gene Ontology enrichment analysis, according to which the lead compounds exert multi-target antiproliferative activity associated with oxidative stress induction and chromatin structure alteration. The betulonic acid diethylentriamine conjugate showed partial activity against methicillin-resistant S. aureus and the fungi C. neoformans. These results show that triterpenic polyamines, being analogs of steroidal squalamine and trodusquemine, are important substances for the search of new drugs with anticancer, antidiabetic, and antimicrobial activities.
Keywords: CellMinner; Gene Ontology; NCI-60 cancer cell line panel; antimicrobial; betulinic acid; betulonic acid; cytotoxicity; lupane triterpenoids; polyamine; spermidine; squalamine; trodusquemine; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Potential targets by pentacyclic triterpenoids from Callicarpa farinosa against methicillin-resistant and sensitive Staphylococcus aureus.Fitoterapia. 2014 Apr;94:48-54. doi: 10.1016/j.fitote.2014.01.026. Epub 2014 Feb 5. Fitoterapia. 2014. PMID: 24508863
-
Antidiabetic and cytotoxic polyhydroxylated oleanane and ursane type triterpenoids from Salvia grossheimii.Bioorg Chem. 2020 Nov;104:104297. doi: 10.1016/j.bioorg.2020.104297. Epub 2020 Sep 19. Bioorg Chem. 2020. PMID: 33011536
-
Inhibition of Alpha-Glucosidase by Synthetic Derivatives of Lupane, Oleanane, Ursane and Dammarane Triterpenoids.Nat Prod Commun. 2016 Jan;11(1):33-5. Nat Prod Commun. 2016. PMID: 26996014
-
Novel targets of pentacyclic triterpenoids in Staphylococcus aureus: A systematic review.Phytomedicine. 2020 Jul 15;73:152933. doi: 10.1016/j.phymed.2019.152933. Epub 2019 Apr 23. Phytomedicine. 2020. PMID: 31103429
-
[Biological activity and pharmacological prospects of lupane terpenoids: I. Natural lupane derivatives].Bioorg Khim. 2006 Jan-Feb;32(1):42-55. doi: 10.1134/s1068162006010031. Bioorg Khim. 2006. PMID: 16523720 Review. Russian.
Cited by
-
Comparison of In Vitro Antimelanoma and Antimicrobial Activity of 2,3-Indolo-betulinic Acid and Its Glycine Conjugates.Plants (Basel). 2023 Mar 9;12(6):1253. doi: 10.3390/plants12061253. Plants (Basel). 2023. PMID: 36986941 Free PMC article.
-
Cuproptosis-related genes and agents: implications in tumor drug resistance and future perspectives.Front Pharmacol. 2025 May 8;16:1559236. doi: 10.3389/fphar.2025.1559236. eCollection 2025. Front Pharmacol. 2025. PMID: 40406488 Free PMC article. Review.
-
Semisynthetic Derivatives of Pentacyclic Triterpenes Bearing Heterocyclic Moieties with Therapeutic Potential.Molecules. 2022 Oct 3;27(19):6552. doi: 10.3390/molecules27196552. Molecules. 2022. PMID: 36235089 Free PMC article. Review.
-
Potentiation effect of mallotojaponin B on chloramphenicol and mode of action of combinations against Methicillin-resistant Staphylococcus aureus.PLoS One. 2023 Mar 21;18(3):e0282008. doi: 10.1371/journal.pone.0282008. eCollection 2023. PLoS One. 2023. PMID: 36943826 Free PMC article.
-
Polyamine-Drug Conjugates: Do They Boost Drug Activity?Molecules. 2023 Jun 2;28(11):4518. doi: 10.3390/molecules28114518. Molecules. 2023. PMID: 37298993 Free PMC article. Review.
References
-
- Salvador J.A.R., Leal A.S., Valdeira A.S., Gonçalves B.M.F., Alho D.P.S., Figueiredo S.A.C., Silvestre S.M., Mendes V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017;142:95–130. doi: 10.1016/j.ejmech.2017.07.013. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

