Recycling the Purpose of Old Drugs to Treat Ovarian Cancer
- PMID: 33092251
- PMCID: PMC7656306
- DOI: 10.3390/ijms21207768
Recycling the Purpose of Old Drugs to Treat Ovarian Cancer
Abstract
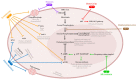
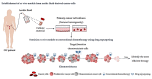
The main challenge in ovarian cancer treatment is the management of recurrences. Facing this scenario, therapy selection is based on multiple factors to define the best treatment sequence. Target therapies, such as bevacizumab and polymerase (PARP) inhibitors, improved patient survival. However, despite their achievements, ovarian cancer survival remains poor; these therapeutic options are highly costly and can be associated with potential side effects. Recently, it has been shown that the combination of repurposed, conventional, chemotherapeutic drugs could be an alternative, presenting good patient outcomes with few side effects and low costs for healthcare institutions. The main aim of this review is to strengthen the importance of repurposed drugs as therapeutic alternatives, and to propose an in vitro model to assess the therapeutic value. Herein, we compiled the current knowledge on the most promising non-oncological drugs for ovarian cancer treatment, focusing on statins, metformin, bisphosphonates, ivermectin, itraconazole, and ritonavir. We discuss the primary drug use, anticancer mechanisms, and applicability in ovarian cancer. Finally, we propose the use of these therapies to perform drug efficacy tests in ovarian cancer ex vivo cultures. This personalized testing approach could be crucial to validate the existing evidences supporting the use of repurposed drugs for ovarian cancer treatment.
Keywords: chemoresistance; drug repurposing; ex vivo cultures; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Current state and outlook for drug repositioning anticipated in the field of ovarian cancer.J Gynecol Oncol. 2019 Jan;30(1):e10. doi: 10.3802/jgo.2019.30.e10. Epub 2018 Oct 10. J Gynecol Oncol. 2019. PMID: 30479094 Free PMC article. Review.
-
Use of "Repurposed" Drugs in the Treatment of Epithelial Ovarian Cancer: A Systematic Review.Am J Clin Oncol. 2022 Apr 1;45(4):168-174. doi: 10.1097/COC.0000000000000900. Am J Clin Oncol. 2022. PMID: 35320817
-
The potential to treat lung cancer via inhalation of repurposed drugs.Adv Drug Deliv Rev. 2018 Aug;133:107-130. doi: 10.1016/j.addr.2018.08.012. Epub 2018 Sep 4. Adv Drug Deliv Rev. 2018. PMID: 30189271 Review.
-
Navigating Metabolic Challenges in Ovarian Cancer: Insights and Innovations in Drug Repurposing.Cancer Med. 2025 Feb;14(4):e70681. doi: 10.1002/cam4.70681. Cancer Med. 2025. PMID: 39969135 Free PMC article. Review.
-
Old wine in new bottles: Drug repurposing in oncology.Eur J Pharmacol. 2020 Jan 5;866:172784. doi: 10.1016/j.ejphar.2019.172784. Epub 2019 Nov 12. Eur J Pharmacol. 2020. PMID: 31730760 Review.
Cited by
-
Combination non-targeted and sGRP78-targeted nanoparticle drug delivery outperforms either component to treat metastatic ovarian cancer.J Control Release. 2024 Nov;375:438-453. doi: 10.1016/j.jconrel.2024.09.014. Epub 2024 Sep 19. J Control Release. 2024. PMID: 39271060
-
Enhancing Immunotherapy in Ovarian Cancer: The Emerging Role of Metformin and Statins.Int J Mol Sci. 2023 Dec 25;25(1):323. doi: 10.3390/ijms25010323. Int J Mol Sci. 2023. PMID: 38203494 Free PMC article. Review.
-
Case Report: Initial Treatment Adjustments and Complications in Ovarian Cancer Patient With Inborn Error of Immunity.Front Oncol. 2022 Jun 29;12:843741. doi: 10.3389/fonc.2022.843741. eCollection 2022. Front Oncol. 2022. PMID: 35847860 Free PMC article.
-
Drug Repurposing Opportunities in Pancreatic Ductal Adenocarcinoma.Pharmaceuticals (Basel). 2021 Mar 20;14(3):280. doi: 10.3390/ph14030280. Pharmaceuticals (Basel). 2021. PMID: 33804613 Free PMC article. Review.
-
Pitavastatin and Ivermectin Enhance the Efficacy of Paclitaxel in Chemoresistant High-Grade Serous Carcinoma.Cancers (Basel). 2022 Sep 7;14(18):4357. doi: 10.3390/cancers14184357. Cancers (Basel). 2022. PMID: 36139522 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

