1-BENZYLSPIRO[PIPERIDINE-4,1'-PYRIDO[3,4-b]indole] 'co-potentiators' for minimal function CFTR mutants
- PMID: 33092904
- PMCID: PMC7744356
- DOI: 10.1016/j.ejmech.2020.112888
1-BENZYLSPIRO[PIPERIDINE-4,1'-PYRIDO[3,4-b]indole] 'co-potentiators' for minimal function CFTR mutants
Abstract
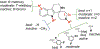
We previously identified a spiro [piperidine-4,1-pyrido [3,4-b]indole] class of co-potentiators that function in synergy with existing CFTR potentiators such as VX-770 or GLGP1837 to restore channel activity of a defined subset of minimal function cystic fibrosis transmembrane conductance regulator (CFTR) mutants. Here, structure-activity studies were conducted to improve their potency over the previously identified compound, 20 (originally termed CP-A01). Targeted synthesis of 37 spiro [piperidine-4,1-pyrido [3,4-b]indoles] was generally accomplished using versatile two or three step reaction protocols with each step having high efficiency. Structure-activity relationship studies established that analog 2i, with 6'-methoxyindole and 2,4,5-trifluorobenzyl substituents, had the greatest potency for activation of N1303K-CFTR, with EC50 ∼600 nM representing an ∼17-fold improvement over the original compound identified in a small molecule screen.
Keywords: CFTR; Cystic fibrosis; Modulator; N1303K-CFTR; Potentiator; c.3700A>G.
Copyright © 2020 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

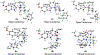

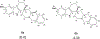


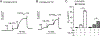

Similar articles
-
Nanomolar-potency 'co-potentiator' therapy for cystic fibrosis caused by a defined subset of minimal function CFTR mutants.Sci Rep. 2019 Nov 27;9(1):17640. doi: 10.1038/s41598-019-54158-2. Sci Rep. 2019. PMID: 31776420 Free PMC article.
-
CFTR modulator therapy for cystic fibrosis caused by the rare c.3700A>G mutation.J Cyst Fibros. 2021 May;20(3):452-459. doi: 10.1016/j.jcf.2020.07.003. Epub 2020 Jul 14. J Cyst Fibros. 2021. PMID: 32674984 Free PMC article.
-
Combination potentiator ('co-potentiator') therapy for CF caused by CFTR mutants, including N1303K, that are poorly responsive to single potentiators.J Cyst Fibros. 2018 Sep;17(5):595-606. doi: 10.1016/j.jcf.2018.05.010. Epub 2018 Jun 12. J Cyst Fibros. 2018. PMID: 29903467 Free PMC article.
-
Elexacaftor/Ivacaftor/Tezacaftor: First Approval.Drugs. 2019 Dec;79(18):2001-2007. doi: 10.1007/s40265-019-01233-7. Drugs. 2019. PMID: 31784874 Review.
-
Corrector combination therapies for F508del-CFTR.Curr Opin Pharmacol. 2017 Jun;34:105-111. doi: 10.1016/j.coph.2017.09.016. Epub 2017 Nov 5. Curr Opin Pharmacol. 2017. PMID: 29080476 Review.
Cited by
-
Pharmacological Modulation of Ion Channels for the Treatment of Cystic Fibrosis.J Exp Pharmacol. 2021 Jul 23;13:693-723. doi: 10.2147/JEP.S255377. eCollection 2021. J Exp Pharmacol. 2021. PMID: 34326672 Free PMC article. Review.
-
Validating organoid-derived human intestinal monolayers for personalized therapy in cystic fibrosis.Life Sci Alliance. 2023 Apr 5;6(6):e202201857. doi: 10.26508/lsa.202201857. Print 2023 Jun. Life Sci Alliance. 2023. PMID: 37024122 Free PMC article.
-
Unraveling the Mechanism of Action, Binding Sites, and Therapeutic Advances of CFTR Modulators: A Narrative Review.Curr Issues Mol Biol. 2025 Feb 11;47(2):119. doi: 10.3390/cimb47020119. Curr Issues Mol Biol. 2025. PMID: 39996840 Free PMC article. Review.
-
Molecular mechanisms of cystic fibrosis - how mutations lead to misfunction and guide therapy.Biosci Rep. 2022 Jul 29;42(7):BSR20212006. doi: 10.1042/BSR20212006. Biosci Rep. 2022. PMID: 35707985 Free PMC article. Review.
References
-
- Elborn JS (2016) Cystic Fibrosis. Lancet 388:2519–2531. - PubMed
-
- Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar and JL VX16-445-001 Study Group (2018) VX-445-Tezacaftor-Ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Engl. J. Med 379:1612–1620. - PMC - PubMed
-
- Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, Mall MA, Welter JJ, Ramsey BW, McKee CM, Marigowda G, Moskowitz SM, Waltz D, Sosnay PR, Simard C, Ahluwalia N, Xuan F, Zhang Y, Taylor-Cousar JL, McCoy and KS V17-445-103 Trial Group (2019) Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomized, phase 3 trial. Lancet 394:1940–1948. - PMC - PubMed
-
- Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D, Ramsey BW, Taylor-Cousar JL, Tullis E, Vermeulen F, Marigowda G, McKee CM, Moskowitz SM, Nair N, Savage J, Simard C, Tian F, Waltz D, Xuan F, Rowe SM, Jain and R V17-445-102 Study Group (2019) Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N. Engl. J. Med 381:1809–1819. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

