1-BENZYLSPIRO[PIPERIDINE-4,1'-PYRIDO[3,4-b]indole] 'co-potentiators' for minimal function CFTR mutants
- PMID: 33092904
- PMCID: PMC7744356
- DOI: 10.1016/j.ejmech.2020.112888
1-BENZYLSPIRO[PIPERIDINE-4,1'-PYRIDO[3,4-b]indole] 'co-potentiators' for minimal function CFTR mutants
Abstract
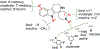
We previously identified a spiro [piperidine-4,1-pyrido [3,4-b]indole] class of co-potentiators that function in synergy with existing CFTR potentiators such as VX-770 or GLGP1837 to restore channel activity of a defined subset of minimal function cystic fibrosis transmembrane conductance regulator (CFTR) mutants. Here, structure-activity studies were conducted to improve their potency over the previously identified compound, 20 (originally termed CP-A01). Targeted synthesis of 37 spiro [piperidine-4,1-pyrido [3,4-b]indoles] was generally accomplished using versatile two or three step reaction protocols with each step having high efficiency. Structure-activity relationship studies established that analog 2i, with 6'-methoxyindole and 2,4,5-trifluorobenzyl substituents, had the greatest potency for activation of N1303K-CFTR, with EC50 ∼600 nM representing an ∼17-fold improvement over the original compound identified in a small molecule screen.
Keywords: CFTR; Cystic fibrosis; Modulator; N1303K-CFTR; Potentiator; c.3700A>G.
Copyright © 2020 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

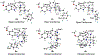

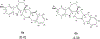


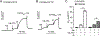

References
-
- Elborn JS (2016) Cystic Fibrosis. Lancet 388:2519–2531. - PubMed
-
- Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar and JL VX16-445-001 Study Group (2018) VX-445-Tezacaftor-Ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Engl. J. Med 379:1612–1620. - PMC - PubMed
-
- Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, Mall MA, Welter JJ, Ramsey BW, McKee CM, Marigowda G, Moskowitz SM, Waltz D, Sosnay PR, Simard C, Ahluwalia N, Xuan F, Zhang Y, Taylor-Cousar JL, McCoy and KS V17-445-103 Trial Group (2019) Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomized, phase 3 trial. Lancet 394:1940–1948. - PMC - PubMed
-
- Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D, Ramsey BW, Taylor-Cousar JL, Tullis E, Vermeulen F, Marigowda G, McKee CM, Moskowitz SM, Nair N, Savage J, Simard C, Tian F, Waltz D, Xuan F, Rowe SM, Jain and R V17-445-102 Study Group (2019) Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N. Engl. J. Med 381:1809–1819. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

