'Be on the TEAM' Study (Teenagers Against Meningitis): protocol for a controlled clinical trial evaluating the impact of 4CMenB or MenB-fHbp vaccination on the pharyngeal carriage of meningococci in adolescents
- PMID: 33093030
- PMCID: PMC7583083
- DOI: 10.1136/bmjopen-2020-037358
'Be on the TEAM' Study (Teenagers Against Meningitis): protocol for a controlled clinical trial evaluating the impact of 4CMenB or MenB-fHbp vaccination on the pharyngeal carriage of meningococci in adolescents
Abstract
Introduction: Capsular group B Neisseria meningitidis (MenB) is the most common cause of invasive meningococcal disease (IMD) in many parts of the world. A MenB vaccine directed against the polysaccharide capsule remains elusive due to poor immunogenicity and safety concerns. The vaccines licensed for the prevention of MenB disease, 4CMenB (Bexsero) and MenB-fHbp (Trumenba), are serogroup B 'substitute' vaccines, comprised of subcapsular proteins and are designed to provide protection against most MenB disease-causing strains. In many high-income countries, such as the UK, adolescents are at increased risk of IMD and have the highest rates of meningococcal carriage. Beginning in the late 1990s, immunisation of this age group with the meningococcal group C conjugate vaccine reduced asymptomatic carriage and disrupted transmission of this organism, resulting in lower group C IMD incidence across all age groups. Whether vaccinating teenagers with the novel 'MenB' protein-based vaccines will prevent acquisition or reduce duration of carriage and generate herd protection was unknown at the time of vaccine introduction and could not be inferred from the effects of the conjugate vaccines. 4CMenB and MenB-fHbp may also impact on non-MenB disease-causing capsular groups as well as commensal Neisseria spp. This study will evaluate the impact of vaccination with 4CMenB or MenB-fHbp on oropharyngeal carriage of pathogenic meningococci in teenagers, and consequently the potential for these vaccines to provide broad community protection against MenB disease.
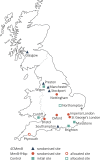
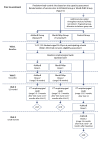
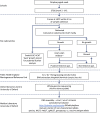
Methods and analysis: The 'Be on the TEAM' (Teenagers Against Meningitis) Study is a pragmatic, partially randomised controlled trial of 24 000 students aged 16-19 years in their penultimate year of secondary school across the UK with regional allocation to a 0+6 month schedule of 4CMenB or MenB-fHbp or to a control group. Culture-confirmed oropharyngeal carriage will be assessed at baseline and at 12 months, following which the control group will be eligible for 4CMenB vaccination. The primary outcome is the carriage prevalence of potentially pathogenic meningococci (defined as those with genogroups B, C, W, Y or X), in each vaccine group compared separately to the control group at 12 months post-enrolment, that is, 12 months after the first vaccine dose and 6 months after the second vaccine dose. Secondary outcomes include impact on carriage of: genogroup B meningococci; hyperinvasive meningococci; all meningococci; those meningococci expressing vaccine antigens and; other Neisseria spp. A sample size of 8000 in each arm will provide 80% power to detect a 30% reduction in meningococcal carriage, assuming genogroup B, C, W, Y or X meningococci carriage of 3.43%, a design effect of 1.5, a retention rate of 80% and a significance level of 0.05. Study results will be available in 2021 and will inform the UK and international immunisation policy and future vaccine development.
Ethics and dissemination: This study is approved by the National Health Service South Central Research Ethics Committee (18/SC/0055); the UK Health Research Authority (IRAS ID 239091) and the UK Medicines and Healthcare products Regulatory Agency. Publications arising from this study will be submitted to peer-reviewed journals. Study results will be disseminated in public forums, online, presented at local and international conferences and made available to the participating schools.
Trial registration numbers: ISRCTN75858406; Pre-results, EudraCT 2017-004609-42.
Keywords: Paediatric infectious disease and immunisation; Paediatric intensive and critical care; infectious diseases; paediatrics.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: AF reports grants from the Department of Health, during the conduct of the study; grants from Pfizer, grants from GSK, outside the submitted work. HC reports other from Sanofi Pasteur, other from IMS Health, grants from National Institute for Health Research, other from AstraZeneca, grants and other from GSK, outside the submitted work. MS reports grants from National Institute for Health Research (NIHR), non-financial support from Pfizer, during the conduct of the study; grants from Pfizer, grants from MCM, grants from GlaxoSmithKline, grants from Novavax, grants from Medimmune, grants from Janssen, outside the submitted work. SC reports salary contribution from the study NIHR grant, during the conduct of the study. SF reports personal fees were paid to his institution (with no personal payment of any kind) from AstraZeneca/Medimmune, Sanofi, Pfizer, Seqirus, Sandoz, Merck, GSK, Alios, Johnson & Johnson and Merck, outside the submitted work. RB reports grants from University of Oxford via a NIHR grant, during the conduct of the study; contract research on behalf of Public Health England for GSK, Pfizer and Sanofi Pasteur, outside the submitted work.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
