Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial)
- PMID: 33093056
- PMCID: PMC7578662
- DOI: 10.1136/bmj.m3939
Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial)
Erratum in
-
Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial).BMJ. 2020 Nov 3;371:m4232. doi: 10.1136/bmj.m4232. BMJ. 2020. PMID: 33144278 Free PMC article. No abstract available.
Abstract
Objective: To investigate the effectiveness of using convalescent plasma to treat moderate coronavirus disease 2019 (covid-19) in adults in India.
Design: Open label, parallel arm, phase II, multicentre, randomised controlled trial.
Setting: 39 public and private hospitals across India.
Participants: 464 adults (≥18 years) admitted to hospital (screened 22 April to 14 July 2020) with confirmed moderate covid-19 (partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2) ratio between 200 mm Hg and 300 mm Hg or a respiratory rate of more than 24/min with oxygen saturation 93% or less on room air): 235 were assigned to convalescent plasma with best standard of care (intervention arm) and 229 to best standard of care only (control arm).
Interventions: Participants in the intervention arm received two doses of 200 mL convalescent plasma, transfused 24 hours apart. The presence and levels of neutralising antibodies were not measured a priori; stored samples were assayed at the end of the study.
Main outcome measure: Composite of progression to severe disease (PaO2/FiO2 <100 mm Hg) or all cause mortality at 28 days post-enrolment.
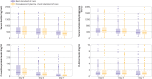
Results: Progression to severe disease or all cause mortality at 28 days after enrolment occurred in 44 (19%) participants in the intervention arm and 41 (18%) in the control arm (risk difference 0.008 (95% confidence interval -0.062 to 0.078); risk ratio 1.04, 95% confidence interval 0.71 to 1.54).
Conclusion: Convalescent plasma was not associated with a reduction in progression to severe covid-19 or all cause mortality. This trial has high generalisability and approximates convalescent plasma use in real life settings with limited laboratory capacity. A priori measurement of neutralising antibody titres in donors and participants might further clarify the role of convalescent plasma in the management of covid-19.
Trial registration: Clinical Trial Registry of India CTRI/2020/04/024775.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare that: TB is a member of the National Task Force for covid-19, which approved the protocol. AM, AA, GK, AT, TB, VS, KK, RS, SD, GD, SS, RG, AS, DP, CP, SS, KJ, HK, PDY, GS, PA, MM, and RMY are employed by the Indian Council of Medical Research (ICMR), the funding source for the trial. PC was an employee of ICMR during the trial.
Figures

Comment in
-
SARS-CoV-2 antibody seroconversion in care home.Nat Rev Immunol. 2020 Nov;20(11):649. doi: 10.1038/s41577-020-00463-1. Nat Rev Immunol. 2020. PMID: 33009507 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
