Barrett's Esophagus and Esophageal Adenocarcinoma Biomarkers
- PMID: 33093162
- PMCID: PMC7710621
- DOI: 10.1158/1055-9965.EPI-20-0223
Barrett's Esophagus and Esophageal Adenocarcinoma Biomarkers
Abstract
Esophageal adenocarcinoma is a major cause of cancer-related morbidity and mortality in Western countries. The incidences of esophageal adenocarcinoma and its precursor Barrett's esophagus have increased substantially in the last four decades. Current care guidelines recommend that endoscopy be used for the early detection and monitoring of patients with Barrett's esophagus; however, the efficacy of this approach is unclear. To prevent the increasing morbidity and mortality from esophageal adenocarcinoma, there is a tremendous need for early detection and surveillance biomarker assays that are accurate, low-cost, and clinically feasible to implement. The last decade has seen remarkable advances in the development of minimally invasive molecular biomarkers, an effort led in large part by the Early Detection Research Network (EDRN). Advances in multi-omics analysis, the development of swallowable cytology collection devices, and emerging technology have led to promising assays that are likely to be implemented into clinical care in the next decade. In this review, an updated overview of the molecular pathology of Barrett's esophagus and esophageal adenocarcinoma and emerging molecular biomarker assays, as well as the role of EDRN in biomarker discovery and validation, will be discussed.See all articles in this CEBP Focus section, "NCI Early Detection Research Network: Making Cancer Detection Possible."
©2020 American Association for Cancer Research.
Conflict of interest statement
Conflicts of Interest:
W. M. Grady is an advisory board member for Freenome, Guardant Health, and SEngine. He is an investigator in a clinical trial sponsored by Janssen Pharmaceuticals. S. D. Markowitz receives income related to patents licensed to Exact Sciences, has founders shares and stock options in LucidDx, serves on the board of directors of LucidDx, serves as a consultant to LucidDx, has sponsored research with LucidDx, and has a royalty interest in patents licensed to LucidDx. A. Chak is an equity holder and advisor for Lucid Diagnostics, consultant for Interpace Diagnostics, nd receives research support from C2Therapeutics/Pentax Inc.
Figures
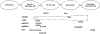
References
-
- American Gastroenterological A, Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91. - PubMed
-
- Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014;63:7–42. - PubMed
-
- Solaymani-Dodaran M, Card TR, West J. Cause-specific mortality of people with Barrett’s esophagus compared with the general population: a population-based cohort study. Gastroenterology 2013;144:1375–83, 1383 e1. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

