Disease-associated mutations in inositol 1,4,5-trisphosphate receptor subunits impair channel function
- PMID: 33093175
- PMCID: PMC7939385
- DOI: 10.1074/jbc.RA120.015683
Disease-associated mutations in inositol 1,4,5-trisphosphate receptor subunits impair channel function
Abstract
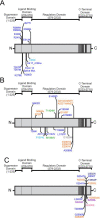
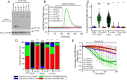
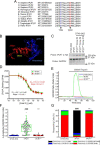
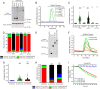
The inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs), which form tetrameric channels, play pivotal roles in regulating the spatiotemporal patterns of intracellular calcium signals. Mutations in IP3Rs have been increasingly associated with many debilitating human diseases such as ataxia, Gillespie syndrome, and generalized anhidrosis. However, how these mutations affect IP3R function, and how the perturbation of as-sociated calcium signals contribute to the pathogenesis and severity of these diseases remains largely uncharacterized. Moreover, many of these diseases occur as the result of autosomal dominant inheritance, suggesting that WT and mutant subunits associate in heterotetrameric channels. How the in-corporation of different numbers of mutant subunits within the tetrameric channels affects its activities and results in different disease phenotypes is also unclear. In this report, we investigated representative disease-associated missense mutations to determine their effects on IP3R channel activity. Additionally, we designed concatenated IP3R constructs to create tetrameric channels with a predefined subunit composition to explore the functionality of heteromeric channels. Using calcium imaging techniques to assess IP3R channel function, we observed that all the mutations studied resulted in severely attenuated Ca2+ release when expressed as homotetramers. However, some heterotetramers retained varied degrees of function dependent on the composition of the tetramer. Our findings suggest that the effect of mutations depends on the location of the mutation in the IP3R structure, as well as on the stoichiometry of mutant subunits assembled within the tetrameric channel. These studies provide insight into the pathogenesis and penetrance of these devastating human diseases.
Keywords: Gillespie syndrome (GS); anhidrosis; calcium channel; calcium imaging; calcium intracellular release; calcium signaling; imaging; inositol 1,4,5-trisphosphate (IP3); inositol 1,4,5-trisphosphate receptor (IP3R); inositol trisphosphate receptor (InsP3R); signal transduction; spinocerebellar ataxia; spinocerebellar ataxia (SCA).
© 2020 Terry et al.
Conflict of interest statement
Conflict of interest—The authors declare no conflicts of interest in regards to the content of this manuscript.
Figures






References
-
- Chin K.V., Cade C., Brostrom C.O., Galuska E.M., Brostrom M.A. Calcium-dependent regulation of protein synthesis at translational initiation in eukaryotic cells. J. Biol. Chem. 1987;262:16509–16514. 3680263. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

