High-amplitude cofluctuations in cortical activity drive functional connectivity
- PMID: 33093200
- PMCID: PMC7668041
- DOI: 10.1073/pnas.2005531117
High-amplitude cofluctuations in cortical activity drive functional connectivity
Abstract
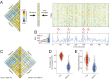
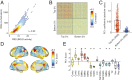
Resting-state functional connectivity is used throughout neuroscience to study brain organization and to generate biomarkers of development, disease, and cognition. The processes that give rise to correlated activity are, however, poorly understood. Here we decompose resting-state functional connectivity using a temporal unwrapping procedure to assess the contributions of moment-to-moment activity cofluctuations to the overall connectivity pattern. This approach temporally resolves functional connectivity at a timescale of single frames, which enables us to make direct comparisons of cofluctuations of network organization with fluctuations in the blood oxygen level-dependent (BOLD) time series. We show that surprisingly, only a small fraction of frames exhibiting the strongest cofluctuation amplitude are required to explain a significant fraction of variance in the overall pattern of connection weights as well as the network's modular structure. These frames coincide with frames of high BOLD activity amplitude, corresponding to activity patterns that are remarkably consistent across individuals and identify fluctuations in default mode and control network activity as the primary driver of resting-state functional connectivity. Finally, we demonstrate that cofluctuation amplitude synchronizes across subjects during movie watching and that high-amplitude frames carry detailed information about individual subjects (whereas low-amplitude frames carry little). Our approach reveals fine-scale temporal structure of resting-state functional connectivity and discloses that frame-wise contributions vary across time. These observations illuminate the relation of brain activity to functional connectivity and open a number of directions for future research.
Keywords: dynamics; functional connectivity; naturalistic stimuli; time-varying connectivity.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Betzel R. F., et al. , Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage 102, 345–357 (2014). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

