Engineered ACE2 receptor traps potently neutralize SARS-CoV-2
- PMID: 33093202
- PMCID: PMC7668070
- DOI: 10.1073/pnas.2016093117
Engineered ACE2 receptor traps potently neutralize SARS-CoV-2
Abstract
An essential mechanism for severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection begins with the viral spike protein binding to the human receptor protein angiotensin-converting enzyme II (ACE2). Here, we describe a stepwise engineering approach to generate a set of affinity optimized, enzymatically inactivated ACE2 variants that potently block SARS-CoV-2 infection of cells. These optimized receptor traps tightly bind the receptor binding domain (RBD) of the viral spike protein and prevent entry into host cells. We first computationally designed the ACE2-RBD interface using a two-stage flexible protein backbone design process that improved affinity for the RBD by up to 12-fold. These designed receptor variants were affinity matured an additional 14-fold by random mutagenesis and selection using yeast surface display. The highest-affinity variant contained seven amino acid changes and bound to the RBD 170-fold more tightly than wild-type ACE2. With the addition of the natural ACE2 collectrin domain and fusion to a human immunoglobulin crystallizable fragment (Fc) domain for increased stabilization and avidity, the most optimal ACE2 receptor traps neutralized SARS-CoV-2-pseudotyped lentivirus and authentic SARS-CoV-2 virus with half-maximal inhibitory concentrations (IC50s) in the 10- to 100-ng/mL range. Engineered ACE2 receptor traps offer a promising route to fighting infections by SARS-CoV-2 and other ACE2-using coronaviruses, with the key advantage that viral resistance would also likely impair viral entry. Moreover, such traps can be predesigned for viruses with known entry receptors for faster therapeutic response without the need for neutralizing antibodies isolated from convalescent patients.
Keywords: SARS-CoV-2; antiviral therapeutics; computational design; receptor trap; yeast display.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: A.G., J.G., I.L., X.X.Z., T.K., and J.A.W. have filed a provisional patent related to this work.
Figures

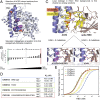
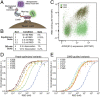


Update of
-
Engineered ACE2 receptor traps potently neutralize SARS-CoV-2.bioRxiv [Preprint]. 2020 Aug 4:2020.07.31.231746. doi: 10.1101/2020.07.31.231746. bioRxiv. 2020. Update in: Proc Natl Acad Sci U S A. 2020 Nov 10;117(45):28046-28055. doi: 10.1073/pnas.2016093117. PMID: 32766586 Free PMC article. Updated. Preprint.
Comment in
-
His345 mutant of angiotensin-converting enzyme 2 (ACE2) remains enzymatically active against angiotensin II.Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2023648118. doi: 10.1073/pnas.2023648118. Proc Natl Acad Sci U S A. 2021. PMID: 33833058 Free PMC article. No abstract available.
-
Reply to Liu et al.: Specific mutations matter in specificity and catalysis in ACE2.Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2024450118. doi: 10.1073/pnas.2024450118. Proc Natl Acad Sci U S A. 2021. PMID: 33833059 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

