Antileukemic activity of the VPS34-IN1 inhibitor in acute myeloid leukemia
- PMID: 33093450
- PMCID: PMC7581748
- DOI: 10.1038/s41389-020-00278-8
Antileukemic activity of the VPS34-IN1 inhibitor in acute myeloid leukemia
Abstract
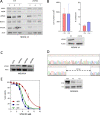
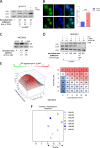
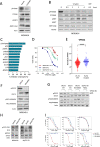
Acute myeloid leukemia (AML) is an aggressive disease with a poor prognosis. Vacuolar protein sorting 34 (VPS34) is a member of the phosphatidylinositol-3-kinase lipid kinase family that controls the canonical autophagy pathway and vesicular trafficking. Using a recently developed specific inhibitor (VPS34-IN1), we found that VPS34 inhibition induces apoptosis in AML cells but not in normal CD34+ hematopoietic cells. Complete and acute inhibition of VPS34 was required for the antileukemic activity of VPS34-IN1. This inhibitor also has pleiotropic effects against various cellular functions related to class III PI3K in AML cells that may explain their survival impairment. VPS34-IN1 inhibits basal and L-asparaginase-induced autophagy in AML cells. A synergistic cell death activity of this drug was also demonstrated. VPS34-IN1 was additionally found to impair vesicular trafficking and mTORC1 signaling. From an unbiased approach based on phosphoproteomic analysis, we identified that VPS34-IN1 specifically inhibits STAT5 phosphorylation downstream of FLT3-ITD signaling in AML. The identification of the mechanisms controlling FLT3-ITD signaling by VPS34 represents an important insight into the oncogenesis of AML and could lead to new therapeutic strategies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

