High-throughput continuous-flow microfluidic electroporation of mRNA into primary human T cells for applications in cellular therapy manufacturing
- PMID: 33093518
- PMCID: PMC7582186
- DOI: 10.1038/s41598-020-73755-0
High-throughput continuous-flow microfluidic electroporation of mRNA into primary human T cells for applications in cellular therapy manufacturing
Abstract
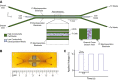
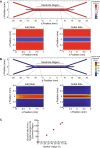
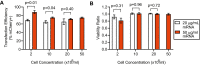
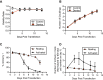
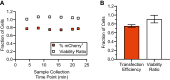
Implementation of gene editing technologies such as CRISPR/Cas9 in the manufacture of novel cell-based therapeutics has the potential to enable highly-targeted, stable, and persistent genome modifications without the use of viral vectors. Electroporation has emerged as a preferred method for delivering gene-editing machinery to target cells, but a major challenge remaining is that most commercial electroporation machines are built for research and process development rather than for large-scale, automated cellular therapy manufacturing. Here we present a microfluidic continuous-flow electrotransfection device designed for precise, consistent, and high-throughput genetic modification of target cells in cellular therapy manufacturing applications. We optimized our device for delivery of mRNA into primary human T cells and demonstrated up to 95% transfection efficiency with minimum impact on cell viability and expansion potential. We additionally demonstrated processing of samples comprising up to 500 million T cells at a rate of 20 million cells/min. We anticipate that our device will help to streamline the production of autologous therapies requiring on the order of 10[Formula: see text]-10[Formula: see text] cells, and that it is well-suited to scale for production of trillions of cells to support emerging allogeneic therapies.
Conflict of interest statement
CAL, JRC, JLB, and VT are listed as inventors on patent applications related to the technology presented. JAS, PH, MW, VLM, ESK, JC, NJH, AGS, AC, DKF, and DAF declare no competing interests.
Figures



References
-
- KYMRIAH (tisagenlecleucel)|Childhood Acute Lymphoblastic Leukemia Treatment. https://www.us.kymriah.com/acute-lymphoblastic-leukemia-children/. Accessed 6 Dec 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

