Anti-staphylococcal activity and mode of action of thioridazine photoproducts
- PMID: 33093568
- PMCID: PMC7582912
- DOI: 10.1038/s41598-020-74752-z
Anti-staphylococcal activity and mode of action of thioridazine photoproducts
Abstract
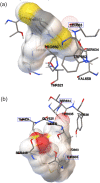
Antibiotic resistance became an increasing risk for population health threatening our ability to fight infectious diseases. The objective of this study was to evaluate the activity of laser irradiated thioridazine (TZ) against clinically-relevant bacteria in view to fight antibiotic resistance. TZ in ultrapure water solutions was irradiated (1-240 min) with 266 nm pulsed laser radiation. Irradiated solutions were characterized by UV-Vis and FTIR absorption spectroscopy, thin layer chromatography, laser-induced fluorescence, and dynamic surface tension measurements. Molecular docking studies were made to evaluate the molecular mechanisms of photoproducts action against Staphylococcus aureus and MRSA. More general, solutions were evaluated for their antimicrobial and efflux inhibitory activity against a panel of bacteria of clinical relevance. We observed an enhanced antimicrobial activity of TZ photoproducts against Gram-positive bacteria. This was higher than ciprofloxacin effects for methicillin- and ciprofloxacin-resistant Staphylococcus aureus. Molecular docking showed the Penicillin-binding proteins PBP3 and PBP2a inhibition by sulforidazine as a possible mechanism of action against Staphylococcus aureus and MRSA strains, respectively. Irradiated TZ reveals possible advantages in the treatment of infectious diseases produced by antibiotic-resistant Gram-positive bacteria. TZ repurposing and its photoproducts, obtained by laser irradiation, show accelerated and low-costs of development if compared to chemical synthesis.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Radhakrishnan V, Ganguly K, Ganguly M, Dastidar SG, Chakrabarty AN. Potentiality of tricyclic compound thioridazine as an effective antibacterial and antiplasmid agent. Indian J. Exp. Biol. 1999;37:671–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

