High colloidal stability ZnO nanoparticles independent on solvent polarity and their application in polymer solar cells
- PMID: 33093600
- PMCID: PMC7582139
- DOI: 10.1038/s41598-020-75070-0
High colloidal stability ZnO nanoparticles independent on solvent polarity and their application in polymer solar cells
Abstract
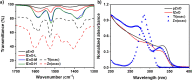
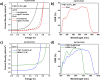
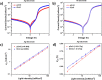
Significant aggregation between ZnO nanoparticles (ZnO NPs) dispersed in polar and nonpolar solvents hinders the formation of high quality thin film for the device application and impedes their excellent electron transporting ability. Herein a bifunctional coordination complex, titanium diisopropoxide bis(acetylacetonate) (Ti(acac)2) is employed as efficient stabilizer to improve colloidal stability of ZnO NPs. Acetylacetonate functionalized ZnO exhibited long-term stability and maintained its superior optical and electrical properties for months aging under ambient atmospheric condition. The functionalized ZnO NPs were then incorporated into polymer solar cells with conventional structure as n-type buffer layer. The devices exhibited nearly identical power conversion efficiency regardless of the use of fresh and old (2 months aged) NPs. Our approach provides a simple and efficient route to boost colloidal stability of ZnO NPs in both polar and nonpolar solvents, which could enable structure-independent intense studies for efficient organic and hybrid optoelectronic devices.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Jr. Miller, P. H. The electrical conductivity of zinc oxide. Phys. Rev.60, 890–895 (1941).
-
- Wang Y, Shi R, Lin J, Zhu Y. Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ. Sci. 2011;4:2922–2929. doi: 10.1039/c0ee00825g. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

