Mechanoregulation in hematopoiesis and hematologic disorders
- PMID: 33094091
- PMCID: PMC7577202
- DOI: 10.1007/s40778-020-00172-4
Mechanoregulation in hematopoiesis and hematologic disorders
Abstract
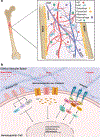
Purpose of review: Hematopoietic stem cells (HSCs) are reliant on intrinsic and extrinsic factors for tight control of self-renewal, quiescence, differentiation, and homing. Given the intimate relationship between HSCs and their niche, increasing numbers of studies are examining how biophysical cues in the hematopoietic microenvironment impact HSC functions.
Recent findings: Numerous mechanosensors are present on hematopoietic cells, including integrins, mechanosensitive ion channels, and primary cilia. Integrin-ligand adhesion, in particular, has been found to be critical for homing and anchoring of HSCs and progenitors in the bone marrow. Integrin-mediated interactions with ligands present on extracellular matrix and endothelial cells are key to establishing long-term engraftment and quiescence of HSCs. Importantly, disruption in the architecture and cellular composition of the bone marrow associated with conditioning regimens and primary myelofibrosis exposes HSCs to a profoundly distinct mechanical environment, with potential implications for progression of hematologic dysfunction and pathologies.
Summary: Study of the mechanobiological signals that govern hematopoiesis represents an important future step toward understanding HSC biology in homeostasis, aging, and cancer.
Keywords: Biomechanical force; hematological disorders; hematopoiesis; hematopoietic stem cells; mechanobiology; mechanosensors.
Conflict of interest statement
Compliance with Ethical Standards Conflict of Interest All authors declare that they have no conflicts of interest.
Figures
References
-
- Ivanovs A, Rybtsov S, Ng ES, Stanley EG, Elefanty AG, Medvinsky A. Human haematopoietic stem cell development : from the embryo to the dish. Development. 2017;144:2323–37. - PubMed
-
-
Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20:303–20.
In this review the authors describe the complex architecture of the bone marrow. It summarizes our current understanding of HSC-niche interactions and the impact of ageing and malignancy on these interactions.
-
-
- Singh AK, McGuirk JP. Allogeneic stem cell transplantation: A historical and scientific overview. Cancer Res. 2016;76:6445–51. - PubMed
-
- Niederwieser D, Pasquini MC, Aljurf MD, Confer DL, Baldomero H, Bouzas LF, et al. Global Hematopoietic Stem Cell Transplantation (HSCT) At One Million: An Achievement Of Pioneers and Foreseeable Challenges For The Next Decade. A Report From The Worldwide Network For Blood and Marrow Transplantation (WBMT). Niederwieser D, editor. Blood. 2013;122:2133 LP – 2133.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
