TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients
- PMID: 33094285
- PMCID: PMC7566418
- DOI: 10.1093/braincomms/fcaa142
TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients
Abstract
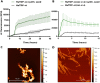
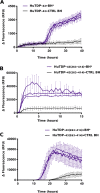
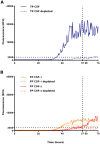
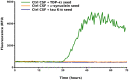
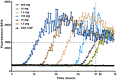
The pathological deposition of the transactive response DNA-binding protein of 43 kDa occurs in the majority (∼97%) of amyotrophic lateral sclerosis and in around 45% of frontotemporal lobar degeneration cases. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration clinically overlap, presenting a continuum of phenotypes. Both amyotrophic lateral sclerosis and frontotemporal lobar degeneration lack treatments capable of interfering with the underlying pathological process and early detection of transactive response DNA-binding protein of 43 kDa pathology would facilitate the development of disease-modifying drugs. The real-time quaking-induced conversion reaction showed the ability to detect prions in several peripheral tissues of patients with different forms of prion and prion-like diseases. Despite transactive response DNA-binding protein of 43 kDa displays prion-like properties, to date the real-time quaking-induced conversion reaction technology has not yet been adapted to this protein. The aim of this study was to adapt the real-time quaking-induced conversion reaction technique for the transactive response DNA-binding protein of 43 kDa substrate and to exploit the intrinsic ability of this technology to amplify minute amount of mis-folded proteins for the detection of pathological transactive response DNA-binding protein of 43 kDa species in the cerebrospinal fluid of amyotrophic lateral sclerosis and frontotemporal lobar degeneration patients. We first optimized the technique with synthetic transactive response DNA-binding protein of 43 kDa-pre-formed aggregates and with autopsy-verified brain homogenate samples and subsequently analysed CSF samples from amyotrophic lateral sclerosis and frontotemporal lobar degeneration patients and controls. Transactive response DNA-binding protein of 43 kDa real-time quaking-induced conversion reaction was able to detect as little as 15 pg of transactive response DNA-binding protein of 43 kDa aggregates, discriminating between a cohort of patients affected by amyotrophic lateral sclerosis and frontotemporal lobar degeneration and age-matched controls with a total sensitivity of 94% and a specificity of 85%. Our data give a proof-of-concept that transactive response DNA-binding protein of 43 kDa is a suitable substrate for the real-time quaking-induced conversion reaction. Transactive response DNA-binding protein of 43 kDa real-time quaking-induced conversion reaction could be an innovative and useful tool for diagnosis and drug development in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. The cerebrospinal fluid detection of transactive response DNA-binding protein of 43 kDa pathological aggregates may be exploited as a disease biomarker for amyotrophic lateral sclerosis and frontotemporal lobar degeneration patients.
Keywords: ALS; FTD; RT-QuIC; TDP-43; biomarker.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al.TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 2006; 351: 602–11. - PubMed
-
- Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, et al.Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 2009; 117: 125–36. - PubMed
-
- Ayers JI, Cashman NR. Prion-like mechanisms in amyotrophic lateral sclerosis. Handb Clin Neurol 2018; 153: 337–54. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
