Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments
- PMID: 33094369
- PMCID: PMC7581469
- DOI: 10.1007/s00216-020-03002-y
Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments
Abstract
The research interest in wearable sensors has tremendously increased in recent years. Amid the different biosensors, electrochemical biosensors are unparalleled and ideal for the design and manufacture of such flexible and wearable sensors because of their various benefits, including convenient operation, quick response, portability, and inherent miniaturization. A number of studies on flexible and wearable electrochemical biosensors have been reported in recent years for invasive/non-invasive and real-time monitoring of biologically relevant molecules such as glucose, lactate, dopamine, cortisol, and antigens. To attain this, novel two-dimensional nanomaterials and their hybrids, various substrates, and detection methods have been explored to fabricate flexible conductive platforms that can be used to develop flexible electrochemical biosensors. In particular, there are many advantages associated with the advent of two-dimensional materials, such as light weight, high stretchability, high performance, and excellent biocompatibility, which offer new opportunities to improve the performance of wearable electrochemical sensors. Therefore, it is urgently required to study wearable/flexible electrochemical biosensors based on two-dimensional nanomaterials for health care monitoring and clinical analysis. In this review, we described recently reported flexible electrochemical biosensors based on two-dimensional nanomaterials. We classified them into specific groups, including enzymatic/non-enzymatic biosensors and affinity biosensors (immunosensors), recent developments in flexible electrochemical immunosensors based on polymer and plastic substrates to monitor biologically relevant molecules. This review will discuss perspectives on flexible electrochemical biosensors based on two-dimensional materials for the clinical analysis and wearable biosensing devices, as well as the limitations and prospects of the these electrochemical flexible/wearable biosensors.Graphical abstract.
Keywords: 2D materials; Biosensor; Electrochemical sensors; Flexible/wearable sensors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





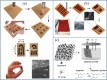







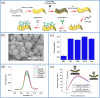




References
-
- Mardonova M, Choi Y. Review of wearable device technology and its applications to the mining industry. Energies. 2018;11(3):547. doi: 10.3390/en11030547. - DOI
-
- Li Y, Fu J, Zhong C, Wu T, Chen Z, Hu W, Amine K, Lu J. Recent Advances inFlexible Zinc-Based Rechargeable Batteries. Adv Energy Mater. 2019;9:1–9. doi: 10.1002/aenm.201802605. - DOI
-
- Tricoli A, Nasiri N, De S. Wearable and Miniaturized Sensor Technologies forPersonalized and Preventive Medicine. Adv Funct Mater. 2017;27:1–19. doi: 10.1002/adfm.201605271. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

