Halogen Complexes of Anionic N-Heterocyclic Carbenes
- PMID: 33094865
- PMCID: PMC7986712
- DOI: 10.1002/chem.202004418
Halogen Complexes of Anionic N-Heterocyclic Carbenes
Abstract
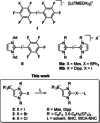
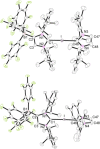
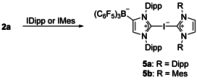
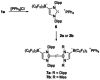
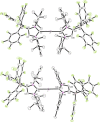
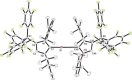
The lithium complexes [(WCA-NHC)Li(toluene)] of anionic N-heterocyclic carbenes with a weakly coordinating anionic borate moiety (WCA-NHC) reacted with iodine, bromine, or CCl4 to afford the zwitterionic 2-halogenoimidazolium borates (WCA-NHC)X (X=I, Br, Cl; WCA=B(C6 F5 )3 , B{3,5-C6 H3 (CF3 )2 }3 ; NHC=IDipp=1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene, or NHC=IMes=1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene). The iodine derivative (WCA-IDipp)I (WCA=B(C6 F5 )3 ) formed several complexes of the type (WCA-IDipp)I⋅L (L=C6 H5 Cl, C6 H5 Me, CH3 CN, THF, ONMe3 ), revealing its ability to act as an efficient halogen bond donor, which was also exploited for the preparation of hypervalent bis(carbene)iodine(I) complexes of the type [(WCA-IDipp)I(NHC)] and [PPh4 ][(WCA-IDipp)I(WCA-NHC)] (NHC=IDipp, IMes). The corresponding bromine complex [PPh4 ][(WCA-IDipp)2 Br] was isolated as a rare example of a hypervalent (10-Br-2) system. DFT calculations reveal that London dispersion contributes significantly to the stability of the bis(carbene)halogen(I) complexes, and the bonding was further analyzed by quantum theory of atoms in molecules (QTAIM) analysis.
Keywords: London dispersion; N-heterocyclic carbenes; halogen bonding; hypervalency; iodine.
© 2020 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Heterobimetallic Coinage Metal-Ruthenium Complexes Supported by Anionic N-Heterocyclic Carbenes.Chemistry. 2021 Nov 2;27(61):15217-15225. doi: 10.1002/chem.202102553. Epub 2021 Sep 3. Chemistry. 2021. PMID: 34342923 Free PMC article.
-
Group 13 Element Trihalide Complexes of Anionic N-Heterocyclic Carbenes.Chem Asian J. 2020 Mar 16;15(6):845-851. doi: 10.1002/asia.201901774. Epub 2020 Feb 20. Chem Asian J. 2020. PMID: 32011782 Free PMC article.
-
Rhodium and Iridium Complexes of Anionic Thione and Selone Ligands Derived from Anionic N-Heterocyclic Carbenes.Chemistry. 2022 Jan 19;28(4):e202104139. doi: 10.1002/chem.202104139. Epub 2021 Dec 22. Chemistry. 2022. PMID: 34878696 Free PMC article.
-
London Dispersion Interactions in Pnictogen Cations [ECl2 ]+ and [E=E]2+ (E=P, As, Sb) Supported by Anionic N-Heterocyclic Carbenes.Chemistry. 2018 Dec 17;24(71):18922-18932. doi: 10.1002/chem.201804714. Epub 2018 Dec 11. Chemistry. 2018. PMID: 30357989
-
Organohypervalent heterocycles.Chem Soc Rev. 2024 May 7;53(9):4786-4827. doi: 10.1039/d2cs01055k. Chem Soc Rev. 2024. PMID: 38545658 Review.
Cited by
-
Metal Centers as Nucleophiles: Oxymoron of Halogen Bond-Involving Crystal Engineering.Chemistry. 2022 Jan 10;28(2):e202103173. doi: 10.1002/chem.202103173. Epub 2021 Oct 29. Chemistry. 2022. PMID: 34623005 Free PMC article. Review.
-
1,3-Bis(tricyanoborane)imidazoline-2-ylidenate Anion-A Ditopic Dianionic N-Heterocyclic Carbene Ligand.Angew Chem Int Ed Engl. 2021 Aug 9;60(33):17974-17980. doi: 10.1002/anie.202105529. Epub 2021 Jun 7. Angew Chem Int Ed Engl. 2021. PMID: 33961330 Free PMC article.
-
Halogen Bond to Experimentally Significant N-Heterocyclic Carbenes (I, IMe2, IiPr2, ItBu2, IPh2, IMes2, IDipp2, IAd2; I = Imidazol-2-ylidene).Int J Mol Sci. 2023 May 21;24(10):9057. doi: 10.3390/ijms24109057. Int J Mol Sci. 2023. PMID: 37240403 Free PMC article.
-
N-Heterocyclic Carbenes Carrying Weakly Coordinating Anions.Chemistry. 2022 Jun 15;28(34):e202200530. doi: 10.1002/chem.202200530. Epub 2022 Apr 27. Chemistry. 2022. PMID: 35357045 Free PMC article.
-
Sydnone Methides-A Forgotten Class of Mesoionic Compounds for the Generation of Anionic N-Heterocyclic Carbenes.Angew Chem Int Ed Engl. 2021 Aug 16;60(34):18882-18887. doi: 10.1002/anie.202107495. Epub 2021 Jul 14. Angew Chem Int Ed Engl. 2021. PMID: 34153173 Free PMC article.
References
-
- None
-
- Waldmann H., Organic Synthesis Highlights II, VCH, Weinheim, 1995;
-
- Stang P. J., Zhdankin V. V., Chem. Rev. 1996, 96, 1123; - PubMed
-
- Zhdankin V. V., Stang P. J., Chem. Rev. 2002, 102, 2523; - PubMed
-
- Wirth T., Kita Y., Hypervalent Iodine Chemistry. Modern Developments in Organic Synthesis, Springer, Berlin, 2003;
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

