Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function: Insights From EMPEROR-Reduced
- PMID: 33095032
- PMCID: PMC7834910
- DOI: 10.1161/CIRCULATIONAHA.120.051685
Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function: Insights From EMPEROR-Reduced
Erratum in
-
Correction to: Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function: Insights from EMPEROR-Reduced.Circulation. 2021 Jan 26;143(4):e29. doi: 10.1161/CIR.0000000000000953. Epub 2021 Jan 25. Circulation. 2021. PMID: 33493037 Free PMC article. No abstract available.
Abstract
Background: In EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), empagliflozin reduced cardiovascular death or heart failure (HF) hospitalization and total HF hospitalizations, and slowed the progressive decline in kidney function in patients with HF and a reduced ejection fraction, with and without diabetes. We aim to study the effect of empagliflozin on cardiovascular and kidney outcomes across the spectrum of kidney function.
Methods: In this prespecified analysis, patients were categorized by the presence or absence of chronic kidney disease (CKD) at baseline (estimated glomerular filtration rate [eGFR] <60 ml/min/1.73 m2 or albumin-to-creatine ratio >300 mg/g). The primary and key secondary outcomes were: (1) a composite of cardiovascular death or HF hospitalization (primary outcome); (2) total HF hospitalizations; and (3) eGFR slope. The direct impact on kidney events was investigated by a prespecified composite kidney outcome (defined as a sustained profound decline in eGFR, chronic dialysis, or transplant). The median follow-up was 16 months.
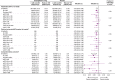
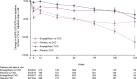
Results: Of 3730 patients who were randomized to empagliflozin or placebo, 1978 (53%) had CKD. Empagliflozin reduced the primary outcome and total HF hospitalizations in patients with and without CKD: hazard ratio (HR)=0.78 (95% CI, 0.65-0.93) and HR=0.72 (95% CI, 0.58-0.90), respectively (interaction P=0.63). Empagliflozin slowed the slope of eGFR decline by 1.11 (0.23-1.98) ml/min/1.73 m2/yr in patients with CKD and by 2.41 (1.49-3.32) ml/min/1.73 m2/yr in patients without CKD. The risk of the composite kidney outcome was reduced similarly in patients with and without CKD: HR=0.53 (95% CI, 0.31-0.91) and HR=0.46 (95% CI, 0.22-0.99), respectively. The effect of empagliflozin on the primary composite outcome and key secondary outcomes was consistent across a broad range of baseline kidney function, measured by clinically relevant eGFR subgroups or by albuminuria, including patients with eGFR as low as 20 ml/min/1.73 m2. Empagliflozin was well tolerated in CKD patients.
Conclusions: In EMPEROR-Reduced, empagliflozin had a beneficial effect on the key efficacy outcomes and slowed the rate of kidney function decline in patients with and without CKD, and regardless of the severity of kidney impairment at baseline. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057977.
Keywords: empagliflozin; glomerular filtration rate; heart failure; renal insufficiency, chronic.
Conflict of interest statement
Dr Zannad reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Janssen, Novartis, Boston Scientific, Amgen, CVRx, AstraZeneca, Vifor Fresenius, Cardior, Cereno Pharmaceutical, Applied Therapeutics, Merck, Bayer, and Cellprothera outside of the submitted work; and other support from cardiovascular clinical trialists and Cardiorenal, outside of the submitted work. Dr Ferreira reports consulting fees from Boehringer Ingelheim during the conduct of the study. Dr Pocock reports personal fees from Boehringer Ingelheim during the conduct of the study. Dr Anker reports grants from Vifor; personal fees from Vifor, Bayer, Boehringer Ingelheim, Novartis, Servier, Impulse Dynamics, Cardiac Dimensions, and Thermo Fisher Scientific; and grants and personal fees from Abbott Vascular, outside of the submitted work. Dr Butler reports consultancy fees from Boehringer Ingelheim during the conduct of the study; and consultancy fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, BerlinCures, Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave, and Vifor, outside of the submitted work. Dr Filippatos reports receiving payment from Boehringer Ingelheim for being a trial committee member during the conduct of the study and from Medtronic, Vifor, Servier, and Novartis for being a trial committee member outside of the submitted work. Dr Wanner reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Akebia, AstraZeneca, Bayer, Eli Lilly, GlaxoSmithKline, Gilead, Merck Sharp & Dohme, Mundipharma, Sanofi-Genzyme, and Vifor Fresenius outside of the submitted work. Dr Packer reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from AbbVie, Akcea, Amarin, AstraZeneca, Amgen, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, Pfizer, Relypsa, Sanofi, Synthetic Biologics, Theravance, and NovoNordisk outside of the submitted work. Drs Hauske, Brueckmann, and Schnee, C. Zeller, and E. Pfarr are employees of Boehringer Ingelheim.
Figures

Comment in
-
Empagliflozin improves kidney outcomes in patients with heart failure and CKD.Nat Rev Nephrol. 2021 Jan;17(1):14. doi: 10.1038/s41581-020-00375-2. Nat Rev Nephrol. 2021. PMID: 33173185 No abstract available.
-
Sodium-Glucose Cotransporter 2 Inhibitors in Patients With Heart Failure With Reduced Ejection Fraction: The Heart and Kidney Working Better Together.Circulation. 2021 Jan 26;143(4):322-325. doi: 10.1161/CIRCULATIONAHA.120.052048. Epub 2021 Jan 25. Circulation. 2021. PMID: 33493029 Free PMC article. No abstract available.
-
In HFrEF, adding empagliflozin to medical therapy reduced a composite outcome, regardless of CKD status.Ann Intern Med. 2021 Jun;174(6):JC68. doi: 10.7326/ACPJ202106150-068. Epub 2021 Jun 1. Ann Intern Med. 2021. PMID: 34058113
References
-
- McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD; Resource Utilization Among Congestive Heart Failure (REACH) Study. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002; 39:60–69. doi: 10.1016/s0735-1097(01)01700-4 - PubMed
-
- Smith DH, Thorp ML, Gurwitz JH, McManus DD, Goldberg RJ, Allen LA, Hsu G, Sung SH, Magid DJ, Go AS. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013; 6:333–342. doi: 10.1161/CIRCOUTCOMES.113.000221 - PMC - PubMed
-
- Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999; 354:359–364. doi: 10.1016/S0140-6736(98)10363-X - PubMed
-
- Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006; 354:131–140. doi: 10.1056/NEJMoa053107 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

