Artemisinin susceptibility in the malaria parasite Plasmodium falciparum: propellers, adaptor proteins and the need for cellular healing
- PMID: 33095255
- PMCID: PMC8100002
- DOI: 10.1093/femsre/fuaa056
Artemisinin susceptibility in the malaria parasite Plasmodium falciparum: propellers, adaptor proteins and the need for cellular healing
Abstract
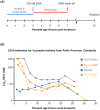
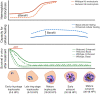
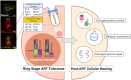
Studies of the susceptibility of Plasmodium falciparum to the artemisinin family of antimalarial drugs provide a complex picture of partial resistance (tolerance) associated with increased parasite survival in vitro and in vivo. We present an overview of the genetic loci that, in mutant form, can independently elicit parasite tolerance. These encode Kelch propeller domain protein PfK13, ubiquitin hydrolase UBP-1, actin filament-organising protein Coronin, also carrying a propeller domain, and the trafficking adaptor subunit AP-2μ. Detailed studies of these proteins and the functional basis of artemisinin tolerance in blood-stage parasites are enabling a new synthesis of our understanding to date. To guide further experimental work, we present two major conclusions. First, we propose a dual-component model of artemisinin tolerance in P. falciparum comprising suppression of artemisinin activation in early ring stage by reducing endocytic haemoglobin capture from host cytosol, coupled with enhancement of cellular healing mechanisms in surviving cells. Second, these two independent requirements limit the likelihood of development of complete artemisinin resistance by P. falciparum, favouring deployment of existing drugs in new schedules designed to exploit these biological limits, thus extending the useful life of current combination therapies.
Keywords: artemisinin resistance; endocytosis; haemoglobin; malaria parasites; proteasome; protein recycling.
© The Author(s) 2020. Published by Oxford University Press on behalf of FEMS.
Figures
Similar articles
-
Local emergence in Amazonia of Plasmodium falciparum k13 C580Y mutants associated with in vitro artemisinin resistance.Elife. 2020 May 12;9:e51015. doi: 10.7554/eLife.51015. Elife. 2020. PMID: 32394893 Free PMC article.
-
Plasmodium berghei K13 Mutations Mediate In Vivo Artemisinin Resistance That Is Reversed by Proteasome Inhibition.mBio. 2020 Nov 10;11(6):e02312-20. doi: 10.1128/mBio.02312-20. mBio. 2020. PMID: 33173001 Free PMC article.
-
Role of Plasmodium falciparum Kelch 13 Protein Mutations in P. falciparum Populations from Northeastern Myanmar in Mediating Artemisinin Resistance.mBio. 2020 Feb 25;11(1):e01134-19. doi: 10.1128/mBio.01134-19. mBio. 2020. PMID: 32098812 Free PMC article.
-
The newly discovered role of endocytosis in artemisinin resistance.Med Res Rev. 2021 Nov;41(6):2998-3022. doi: 10.1002/med.21848. Epub 2021 Jul 26. Med Res Rev. 2021. PMID: 34309894 Review.
-
Artemisinin Action and Resistance in Plasmodium falciparum.Trends Parasitol. 2016 Sep;32(9):682-696. doi: 10.1016/j.pt.2016.05.010. Epub 2016 Jun 9. Trends Parasitol. 2016. PMID: 27289273 Free PMC article. Review.
Cited by
-
Plasmodium falciparum resistance to ACTs: Emergence, mechanisms, and outlook.Int J Parasitol Drugs Drug Resist. 2021 Aug;16:102-118. doi: 10.1016/j.ijpddr.2021.05.007. Epub 2021 May 26. Int J Parasitol Drugs Drug Resist. 2021. PMID: 34090067 Free PMC article.
-
Drug resistance-associated mutations in Plasmodium UBP-1 disrupt its essential deubiquitinating activity.J Biol Chem. 2025 Mar;301(3):108266. doi: 10.1016/j.jbc.2025.108266. Epub 2025 Feb 3. J Biol Chem. 2025. PMID: 39909372 Free PMC article.
-
Assessment of Plasmodium falciparum Artemisinin Resistance Independent of kelch13 Polymorphisms and with Escalating Malaria in Bangladesh.mBio. 2022 Feb 22;13(1):e0344421. doi: 10.1128/mbio.03444-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35073756 Free PMC article.
-
Comparison of silver and gold nanoparticles green synthesis by Artemisia annua hairy root extracts.Biol Open. 2025 Mar 15;14(3):bio061739. doi: 10.1242/bio.061739. Epub 2025 Mar 19. Biol Open. 2025. PMID: 40067211 Free PMC article.
-
In vitro delayed response to dihydroartemisinin of malaria parasites infecting sickle cell erythocytes.Malar J. 2024 Jan 4;23(1):9. doi: 10.1186/s12936-023-04819-5. Malar J. 2024. PMID: 38178227 Free PMC article.
References
-
- Abu-Bakar N, Klonis N, Hanssen Eet al. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J Cell Sci. 2010;123:441–50. - PubMed

