T reg cell-intrinsic requirements for ST2 signaling in health and neuroinflammation
- PMID: 33095261
- PMCID: PMC7590508
- DOI: 10.1084/jem.20201234
T reg cell-intrinsic requirements for ST2 signaling in health and neuroinflammation
Abstract
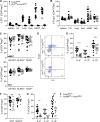
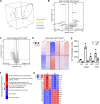
ST2, the receptor for the alarmin IL-33, is expressed by a subset of regulatory T (T reg) cells residing in nonlymphoid tissues, and these cells can potently expand upon provision of exogenous IL-33. Whether the accumulation and residence of T reg cells in tissues requires their cell-intrinsic expression of and signaling by ST2, or whether indirect IL-33 signaling acting on other cells suffices, has been a matter of contention. Here, we report that ST2 expression on T reg cells is largely dispensable for their accumulation and residence in nonlymphoid organs, including the visceral adipose tissue (VAT), even though cell-intrinsic sensing of IL-33 promotes type 2 cytokine production by VAT-residing T reg cells. In addition, we uncovered a novel ST2-dependent role for T reg cells in limiting the size of IL-17A-producing γδT cells in the CNS in a mouse model of neuroinflammation, experimental autoimmune encephalomyelitis (EAE). Finally, ST2 deficiency limited to T reg cells led to disease exacerbation in EAE.
© 2020 Hemmers et al.
Conflict of interest statement
Disclosures: A.Y. Rudensky reported personal fees from Sonoma Biotherapeutics outside the submitted work. No other disclosures were reported.
Figures






References
-
- Allan, D., Fairlie-Clarke K.J., Elliott C., Schuh C., Barnett S.C., Lassmann H., Linnington C., and Jiang H.R.. 2016. Role of IL-33 and ST2 signalling pathway in multiple sclerosis: expression by oligodendrocytes and inhibition of myelination in central nervous system. Acta Neuropathol. Commun. 4:75 10.1186/s40478-016-0344-1 - DOI - PMC - PubMed
-
- Bennett, C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., and Ochs H.D.. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21. 10.1038/83713 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

