Large-Scale Multi-omic Analysis of COVID-19 Severity
- PMID: 33096026
- PMCID: PMC7543711
- DOI: 10.1016/j.cels.2020.10.003
Large-Scale Multi-omic Analysis of COVID-19 Severity
Abstract
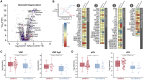
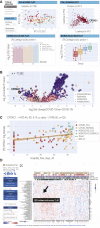
We performed RNA-seq and high-resolution mass spectrometry on 128 blood samples from COVID-19-positive and COVID-19-negative patients with diverse disease severities and outcomes. Quantified transcripts, proteins, metabolites, and lipids were associated with clinical outcomes in a curated relational database, uniquely enabling systems analysis and cross-ome correlations to molecules and patient prognoses. We mapped 219 molecular features with high significance to COVID-19 status and severity, many of which were involved in complement activation, dysregulated lipid transport, and neutrophil activation. We identified sets of covarying molecules, e.g., protein gelsolin and metabolite citrate or plasmalogens and apolipoproteins, offering pathophysiological insights and therapeutic suggestions. The observed dysregulation of platelet function, blood coagulation, acute phase response, and endotheliopathy further illuminated the unique COVID-19 phenotype. We present a web-based tool (covid-omics.app) enabling interactive exploration of our compendium and illustrate its utility through a machine learning approach for prediction of COVID-19 severity.
Keywords: ARDS; COVID-19; ICU; RNA sequencing; machine learning; mass spectrometry; multi-omics; outcomes; severity.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





Update of
-
Large-scale Multi-omic Analysis of COVID-19 Severity.medRxiv [Preprint]. 2020 Jul 19:2020.07.17.20156513. doi: 10.1101/2020.07.17.20156513. medRxiv. 2020. Update in: Cell Syst. 2021 Jan 20;12(1):23-40.e7. doi: 10.1016/j.cels.2020.10.003. PMID: 32743614 Free PMC article. Updated. Preprint.
References
-
- Arndt S., Turvey C., Andreasen N.C. Correlating and predicting psychiatric symptom ratings: Spearman’s R versus Kendall's Tau correlation. J. Psychiatr. Res. 1999;33:97–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

