An Acquired and Endogenous Glycocalyx Forms a Bidirectional "Don't Eat" and "Don't Eat Me" Barrier to Phagocytosis
- PMID: 33096038
- PMCID: PMC7855322
- DOI: 10.1016/j.cub.2020.09.082
An Acquired and Endogenous Glycocalyx Forms a Bidirectional "Don't Eat" and "Don't Eat Me" Barrier to Phagocytosis
Abstract
Macrophages continuously survey their environment in search of pathogens or apoptotic corpses or debris. Targets intended for clearance expose ligands that initiate their phagocytosis ("eat me" signals), while others avoid phagocytosis by displaying inhibitory ligands ("don't eat me" signals). We report that such ligands can be obscured by the glycosaminoglycans and glycoproteins that coat pathogenic as well as malignant phagocytic targets. In addition, a reciprocal barrier of self-synthesized or acquired glycocalyx components on the macrophage surface shrouds phagocytic receptors, curtailing their ability to engage particles. The coating layers of macrophages and their targets hinder phagocytosis by both steric and electrostatic means. Their removal by enzymatic means is shown to markedly enhance phagocytic efficiency. In particular, we show that the removal of mucins, which are overexpressed in cancer cells, facilitates their clearance. These results shed light on the physical barriers that modulate phagocytosis, which have been heretofore underappreciated. VIDEO ABSTRACT.
Keywords: CD47; Candida albicans; hyaluronan; mucinase; sialic acid; surface charge; syndecan; synovial-resident macrophages; tumor-associated macrophages.
Crown Copyright © 2020. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests C.R.B is a co-founder and scientific advisory board member of Lycia Therapeutics, Palleon Pharmaceuticals, Enable Bioscience, Redwood Biosciences (a subsidiary of Catalent), and InterVenn Biosciences, and a member of the board of directors of Eli Lilly & Company.
Figures
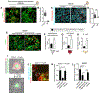

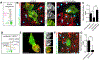
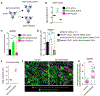
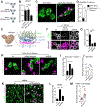
Comment in
-
Phagocytosis: Sweet Repulsions via the Glycocalyx.Curr Biol. 2021 Jan 11;31(1):R20-R22. doi: 10.1016/j.cub.2020.10.066. Curr Biol. 2021. PMID: 33434480
References
-
- Flannagan RS, Jaumouille V, and Grinstein S (2012). The cell biology of phagocytosis. Annu Rev Pathol 7, 61–98. - PubMed
-
- Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, et al. (2013). Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A 110, 11103–11108. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

