Preventing the development of severe COVID-19 by modifying immunothrombosis
- PMID: 33096114
- PMCID: PMC7574725
- DOI: 10.1016/j.lfs.2020.118617
Preventing the development of severe COVID-19 by modifying immunothrombosis
Abstract
Background: COVID-19-associated acute respiratory distress syndrome (ARDS) is associated with significant morbidity and high levels of mortality. This paper describes the processes involved in the pathophysiology of COVID-19 from the initial infection and subsequent destruction of type II alveolar epithelial cells by SARS-CoV-2 and culminating in the development of ARDS.
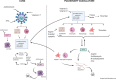
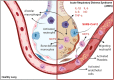
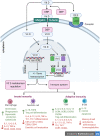
Main body: The activation of alveolar cells and alveolar macrophages leads to the release of large quantities of proinflammatory cytokines and chemokines and their translocation into the pulmonary vasculature. The presence of these inflammatory mediators in the vascular compartment leads to the activation of vascular endothelial cells platelets and neutrophils and the subsequent formation of platelet neutrophil complexes. These complexes in concert with activated endothelial cells interact to create a state of immunothrombosis. The consequence of immunothrombosis include hypercoagulation, accelerating inflammation, fibrin deposition, migration of neutrophil extracellular traps (NETs) producing neutrophils into the alveolar apace, activation of the NLRP3 inflammazome, increased alveolar macrophage destruction and massive tissue damage by pyroptosis and necroptosis Therapeutic combinations aimed at ameliorating immunothrombosis and preventing the development of severe COVID-19 are discussed in detail.
Keywords: COVID-19; Respiratory infection; SARS-CoV-2; Treatment.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
MB is supported by a NHMRC Senior Principal Research Fellowship (1059660 and 1156072). MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Australian Rotary Health, A2 Milk Company, Meat and Livestock Australia, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Astra Zeneca, Lundbeck, Merck, Pfizer, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier – all unrelated to this work. LO is supported by a NHMRC Early Career Fellowship (1158487). WM is currently funded by an Alfred Deakin Postdoctoral Research Fellowship and a Multiple Sclerosis Research Australia early-career fellowship. WM has previously received funding from the Cancer Council Queensland and university grants/fellowships from La Trobe University, Deakin University, University of Queensland, and Bond University. WM has received industry funding and has attended events funded by Cobram Estate Pty. Ltd. WM has received travel funding from Nutrition Society of Australia. WM has received consultancy funding from Nutrition Research Australia. WM has received speaker honoraria from The Cancer Council Queensland and the Princess Alexandra Research Foundation. The Food & Mood Centre has received Grant/Research support from Fernwood Foundation, Wilson Foundation, the A2 Milk Company, and Be Fit Foods. AO is supported by a Future Leader Fellowship (#101160) from the Heart Foundation Australia and Wilson Foundation. She has received research funding from National Health and Medical Research Council, Australian Research Council, University of Melbourne, Deakin University, Sanofi, Meat and Livestock Australia and Woolworths Limited and Honoraria from Novartis.
Figures
Similar articles
-
The pathophysiology of SARS-CoV-2: A suggested model and therapeutic approach.Life Sci. 2020 Oct 1;258:118166. doi: 10.1016/j.lfs.2020.118166. Epub 2020 Jul 31. Life Sci. 2020. PMID: 32739471 Free PMC article. Review.
-
Pathomechanisms Underlying Hypoxemia in Two COVID-19-Associated Acute Respiratory Distress Syndrome Phenotypes: Insights From Thrombosis and Hemostasis.Shock. 2022 Jan 1;57(1):1-6. doi: 10.1097/SHK.0000000000001825. Shock. 2022. PMID: 34172612 Free PMC article. Review.
-
Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-Kb pathway deregulation: A physio-pathological theory.Med Hypotheses. 2021 Jan;146:110412. doi: 10.1016/j.mehy.2020.110412. Epub 2020 Nov 23. Med Hypotheses. 2021. PMID: 33308936 Free PMC article.
-
CLEC5A and TLR2 are critical in SARS-CoV-2-induced NET formation and lung inflammation.J Biomed Sci. 2022 Jul 11;29(1):52. doi: 10.1186/s12929-022-00832-z. J Biomed Sci. 2022. PMID: 35820906 Free PMC article.
-
Neutrophil activation and neutrophil extracellular traps (NETs) in COVID-19 ARDS and immunothrombosis.Eur J Immunol. 2023 Jan;53(1):e2250010. doi: 10.1002/eji.202250010. Epub 2022 Nov 1. Eur J Immunol. 2023. PMID: 36239164 Free PMC article. Review.
Cited by
-
Biomarkers during COVID-19: Mechanisms of Change and Implications for Patient Outcomes.Diagnostics (Basel). 2022 Feb 16;12(2):509. doi: 10.3390/diagnostics12020509. Diagnostics (Basel). 2022. PMID: 35204599 Free PMC article. Review.
-
Use of Thiols in the Treatment of COVID-19: Current Evidence.Lung. 2021 Aug;199(4):335-343. doi: 10.1007/s00408-021-00465-3. Epub 2021 Aug 27. Lung. 2021. PMID: 34448938 Free PMC article. Review.
-
Soluble CD13 is a potential mediator of neutrophil-induced thrombogenic inflammation in SARS-CoV-2 infection.JCI Insight. 2025 Apr 1;10(9):e184975. doi: 10.1172/jci.insight.184975. eCollection 2025 May 8. JCI Insight. 2025. PMID: 40168094 Free PMC article.
-
The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all?Cytokine. 2021 Aug;144:155593. doi: 10.1016/j.cyto.2021.155593. Epub 2021 May 26. Cytokine. 2021. PMID: 34074585 Free PMC article. Review.
-
Molecular mechanisms and roles of pyroptosis in acute lung injury.Chin Med J (Engl). 2022 Oct 20;135(20):2417-2426. doi: 10.1097/CM9.0000000000002425. Chin Med J (Engl). 2022. PMID: 36583860 Free PMC article.
References
-
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

