The requirement for cobalt in vitamin B12: A paradigm for protein metalation
- PMID: 33096143
- PMCID: PMC7689651
- DOI: 10.1016/j.bbamcr.2020.118896
The requirement for cobalt in vitamin B12: A paradigm for protein metalation
Abstract
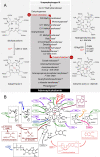
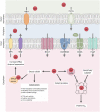
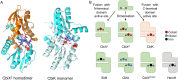
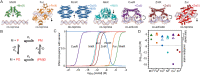
Vitamin B12, cobalamin, is a cobalt-containing ring-contracted modified tetrapyrrole that represents one of the most complex small molecules made by nature. In prokaryotes it is utilised as a cofactor, coenzyme, light sensor and gene regulator yet has a restricted role in assisting only two enzymes within specific eukaryotes including mammals. This deployment disparity is reflected in another unique attribute of vitamin B12 in that its biosynthesis is limited to only certain prokaryotes, with synthesisers pivotal in establishing mutualistic microbial communities. The core component of cobalamin is the corrin macrocycle that acts as the main ligand for the cobalt. Within this review we investigate why cobalt is paired specifically with the corrin ring, how cobalt is inserted during the biosynthetic process, how cobalt is made available within the cell and explore the cellular control of cobalt and cobalamin levels. The partitioning of cobalt for cobalamin biosynthesis exemplifies how cells assist metalation.
Keywords: Chelation; Cobalamin; Cobamide; Homeostasis; Metals; sensors.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Whipple G.H., Hooper C.W., Robscheit F.S. Blood regeneration following anemia, iv: in- fluence of meat, liver and various extrac- tives, alone or combined with standard diets. JAMA. 1920;53:236–262.
-
- Minot G.R., Murphy W.P. Treatment of perni- cious anemia by a special diet. JAMA. 1926;87:470–476.
-
- Shorb M.S. Activity of vitamin B12 for the growth of Lactobacillus lactis. Science. 1948;107(2781):397–398. - PubMed
-
- Rickes E.L., Brink N.G., Koniuszy F.R., Wood T.R., Folkers K. Crystalline vitamin B12. Science. 1948;107(2781):396–397. - PubMed
-
- Smith E.L. Purification of anti-pernicious anaemia factors from liver. Nature. 1948;161(4095):638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

