High-throughput genotyping of high-homology mutant mouse strains by next-generation sequencing
- PMID: 33096238
- PMCID: PMC8205115
- DOI: 10.1016/j.ymeth.2020.10.011
High-throughput genotyping of high-homology mutant mouse strains by next-generation sequencing
Abstract
Genotyping of knockout alleles in mice is commonly performed by end-point PCR or gene-specific/universal cassette qPCR. Both have advantages and limitations in terms of assay design and interpretation of results. As an alternative method for high-throughput genotyping, we investigated next generation sequencing (NGS) of PCR amplicons, with a focus on CRISPR-mediated exon deletions where antibiotic selection markers are not present. By multiplexing the wild type and mutant-specific PCR reactions, the genotype can be called by the relative sequence counts of each product. The system is highly scalable and can be applied to a variety of different allele types, including those produced by the International Mouse Phenotyping Consortium and associated projects. One potential challenge with any assay design is locating unique areas of the genome, especially when working with gene families or regions of high homology. These can result in misleading or ambiguous genotypes for either qPCR or end-point assays. Here, we show that genotyping by NGS can negate these issues by simple, automated filtering of undesired sequences. Analysis and genotype calls can also be fully automated, using FASTQ or FASTA input files and an in-house Perl script and SQL database.
Keywords: CRISPR; Genotyping; Mouse; Mutant; NGS; QC.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


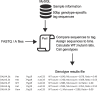
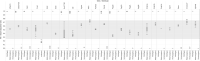
References
-
- Cacheiro P., Haendel M.A., Smedley D., Meehan T., Mason J., Mashhadi H.H., Muñoz-Fuentes V., Tocchini G., Lloyd K.K.C., McKerlie C., Bower L., Clary D., Nutter L.M.J., Flenniken A.M., Teboul L., Codner G., Wells S., Herault Y., Sorg T., Vasseurm L., Selloum M., Roux M., Jacobs H., Meziane H., Champy M.F., About G.B., Murray S., Chesler E., Kumar V., White J., Braun R.E., Beaudet A.L., Dickinson M.E., Heaney J.D., Lorenzo I., Lanza D.G., Reynolds C.L., Ward C.S., Samaco R.C., Veeraragavan S., Hsu C.W., Christianson A.E., Gallegos J.J., Seavitt J.R., Gaspero A., Green J.R., Garza A., Garza R., Bohat R., Sedlacek S.D.M.B. New models for human disease from the International Mouse Phenotyping Consortium. Mamm. Genome. 2019;30:143–150. doi: 10.1007/s00335-019-09804-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

