Aquaporins in lung health and disease: Emerging roles, regulation, and clinical implications
- PMID: 33096317
- PMCID: PMC7733530
- DOI: 10.1016/j.rmed.2020.106193
Aquaporins in lung health and disease: Emerging roles, regulation, and clinical implications
Abstract
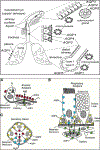
Aquaporins (AQPs) aka water channels are a family of conserved transmembrane proteins (~30 kDa monomers) expressed in various organ systems. Of the 13 AQPs (AQP0 through AQP12) in the human body, four (AQPs 1, 3, 4, and 5) are expressed in the respiratory system. These channels are conventionally known for mediating transcellular fluid movements. Certain AQPs (aquaglyceroporins) have the capability to transport glycerol and potentially other solutes. There is an emerging body of literature unveiling the non-conventional roles of AQPs such as in cell proliferation and migration, gas permeation, signal potentiation, etc. Initial gene knock-out studies established a physiological role for lung AQPs, particularly AQP5, in maintaining homeostasis, by mediating fluid secretion from submucosal glands onto the airway surface liquid (ASL) lining. Subsequent studies have highlighted the functional significance of AQPs, particularly AQP1 and AQP5 in lung pathophysiology and diseases, including but not limited to chronic and acute lung injury, chronic obstructive pulmonary disease (COPD), other inflammatory lung conditions, and lung cancer. AQP1 has been suggested as a potential prognostic marker for malignant mesothelioma. Recent efforts are directed toward exploiting AQPs as targets for diagnosis, prevention, intervention, and/or treatment of various lung conditions. Emerging information on regulatory pathways and directed mechanistic research are posited to unravel novel strategies for these clinical implications. Future considerations should focus on development of AQP inhibitors, blockers, and modulators for therapeutic needs, and better understanding the role of lung-specific AQPs in inter-individual susceptibility to chronic lung diseases such as COPD and cancer.
Keywords: Acute lung injury; Aquaporins; Chronic obstructive pulmonary disease; Lung cancer; Lung inflammation; Lung pathophysiology; Regulation.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

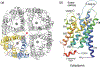
Similar articles
-
Aquaporins in sepsis- an update.Front Immunol. 2024 Oct 31;15:1495206. doi: 10.3389/fimmu.2024.1495206. eCollection 2024. Front Immunol. 2024. PMID: 39544938 Free PMC article. Review.
-
Aquaporins in Respiratory System.Adv Exp Med Biol. 2017;969:115-122. doi: 10.1007/978-94-024-1057-0_7. Adv Exp Med Biol. 2017. PMID: 28258569 Review.
-
More than just water channels: unexpected cellular roles of aquaporins.J Cell Sci. 2005 Aug 1;118(Pt 15):3225-32. doi: 10.1242/jcs.02519. J Cell Sci. 2005. PMID: 16079275 Review.
-
Aquaporins in Respiratory System.Adv Exp Med Biol. 2023;1398:137-144. doi: 10.1007/978-981-19-7415-1_9. Adv Exp Med Biol. 2023. PMID: 36717491
-
Lung edema clearance: 20 years of progress: invited review: role of aquaporin water channels in fluid transport in lung and airways.J Appl Physiol (1985). 2002 Dec;93(6):2199-206. doi: 10.1152/japplphysiol.01171.2001. J Appl Physiol (1985). 2002. PMID: 12433939 Review.
Cited by
-
Lactobacillus reuteri Alleviates Hyperoxia-Induced BPD by Activating IL-22/STAT3 Signaling Pathway in Neonatal Mice.Mediators Inflamm. 2024 Dec 9;2024:4965271. doi: 10.1155/mi/4965271. eCollection 2024. Mediators Inflamm. 2024. PMID: 39687635 Free PMC article.
-
Gandan Oral Liquid Improves Exudative Pneumonia by Upregulating Bacteria Clearance via Regulating AQP5 and MUC5AC in Rats.Evid Based Complement Alternat Med. 2022 Apr 26;2022:3890347. doi: 10.1155/2022/3890347. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35518345 Free PMC article.
-
Distinctive roles of aquaporins and novel therapeutic opportunities against cancer.RSC Med Chem. 2024 Dec 17. doi: 10.1039/d4md00786g. Online ahead of print. RSC Med Chem. 2024. PMID: 39697243 Free PMC article. Review.
-
Topology-driven discovery of transmembrane protein S-palmitoylation.J Biol Chem. 2025 Mar;301(3):108259. doi: 10.1016/j.jbc.2025.108259. Epub 2025 Feb 3. J Biol Chem. 2025. PMID: 39909380 Free PMC article.
-
Role of aquaporins in corneal healing post chemical injury.Exp Eye Res. 2023 Mar;228:109390. doi: 10.1016/j.exer.2023.109390. Epub 2023 Jan 22. Exp Eye Res. 2023. PMID: 36696947 Free PMC article. Review.
References
-
- Yool AJ, Brown EA, Flynn GA, Roles for novel pharmacological blockers of aquaporins in the treatment of brain oedema and cancer, Clin Exp Pharmacol Physiol 37(4) (2010) 403–9. - PubMed
-
- Borok Z, Verkman AS, Lung edema clearance: 20 years of progress: invited review: role of aquaporin water channels in fluid transport in lung and airways, J Appl Physiol (1985) 93(6) (2002) 2199–206. - PubMed
-
- Conner AC, Bill RM, Conner MT, An emerging consensus on aquaporin translocation as a regulatory mechanism, Mol Membr Biol 30(1) (2013) 1–12. - PubMed
-
- Gonen T, Walz T, The structure of aquaporins, Q Rev Biophys 39(4) (2006) 361–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

