Oral commensal bacteria differentially modulate epithelial cell death
- PMID: 33096404
- PMCID: PMC7655725
- DOI: 10.1016/j.archoralbio.2020.104926
Oral commensal bacteria differentially modulate epithelial cell death
Abstract
Objective: Epithelial cell death is an important innate mechanism at mucosal surfaces, which enables the elimination of pathogens and modulates immunoinflammatory responses. Based on the antimicrobial and anti-inflammatory properties of cell death, we hypothesized that oral epithelial cell (OECs) death is differentially modulated by oral bacteria.
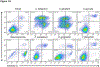
Material and methods: We evaluated the effect of oral commensals Streptococcus gordonii (Sg), Streptococcus sanguinis (Ss), and Veillonella parvula (Vp), and pathogens Porphyromonas gingivalis (Pg), Tannerella forsythia (Tf), and Fusobacterium nucleatum (Fn) on OEC death. Apoptosis and necrosis were evaluated by flow cytometry using FITC Annexin-V and Propidium Iodide staining. Caspase-3/7 and caspase-1 activities were determined as markers of apoptosis and pyroptosis, respectively. IL-1β and IL-8 protein levels were determined in supernatants by ELISA.
Results: Significant increases in apoptosis and necrosis were induced by Sg and Ss. Pg also induced apoptosis, although at a substantially lower level than the commensals. Vp, Tf, and Fn showed negligible effects on cell viability. These results were consistent with Sg, Ss, and Pg activating caspase-3/7. Only Ss significantly increased the levels of activated caspase-1, which correlated to IL-1β over-expression.
Conclusions: OEC death processes were differentially induced by oral commensal and pathogenic bacteria, with Sg and Ss being more pro-apoptotic and pro-pyroptotic than pathogenic bacteria. Oral commensal-induced cell death may be a physiological mechanism to manage the extent of bacterial colonization of the outer layers of mucosal epithelial surfaces. Dysbiosis-related reduction or elimination of pro-apoptotic oral bacterial species could contribute to the risk for persistent inflammation and tissue destruction.
Keywords: Apoptosis; Oral commensal bacteria; Oral epithelial cells; Oral pathogenic bacteria; Pyroptosis.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no potential conflicts of interest with respect to authorship and/or publication of this manuscript.
Figures





References
-
- Al-Attar A, Alimova Y, Kirakodu S, Kozal A, Novak MJ, Stromberg AJ, Orraca L, Gonzalez-Martinez J, Martinez M, Ebersole JL, & Gonzalez OA (2018). Activation of Notch-1 in oral epithelial cells by P. gingivalis triggers the expression of the antimicrobial protein PLA2-IIA. Mucosal Immunol, 11(4), 1047–1059. 10.1038/s41385-018-0014-7 - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

