Epistatic evidence for gender-dependant slow neurotransmission signalling in substance use disorders: PPP1R12B versus PPP1R1B
- PMID: 33096475
- PMCID: PMC7581882
- DOI: 10.1016/j.ebiom.2020.103066
Epistatic evidence for gender-dependant slow neurotransmission signalling in substance use disorders: PPP1R12B versus PPP1R1B
Abstract
Background: Slow neurotransmission including DARPP-32 signalling is implicated in substance use disorders (SUDs) by experimental systems but not yet in the human aetiology. PPP1R12B, encoding another protein in the DARPP-32 family, hasn't been studied in the brain.
Methods: Brain-regional gene activity was assessed in three different animal models of SUDs for mRNA level alterations. Genetic associations were assessed by meta-analysis of pre-existing dbGaP GWAS datasets for main effects and epistasis with known genetic risks, followed by cell type-specific pathway delineation. Parkinson's disease (PD) was included as a dopamine-related disease control for SUDs.
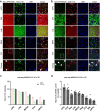
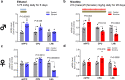
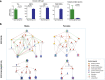
Findings: In animal models of SUDs, environmentally-altered PPP1R12B expression sex-dependently involves motivation-related brain regions. In humans with polysubstance abuse, meta-analysis of pre-existing datasets revealed that PPP1R12B and PPP1R1B, although expressed in dopamine vs. dopamine-recipient neurons, exerted similar interactions with known genetic risks such as ACTR1B and DRD2 in men but with ADH1B, HGFAC and DRD3 in women. These interactions reached genome-wide significances (Pmeta<10-20) for SUDs but not for PD (disease selectivity: P = 4.8 × 10-142, OR = 6.7 for PPP1R12B; P = 8.0 × 10-8, OR = 2.1 for PPP1R1B). CADM2 was the common risk in the molecular signalling regardless of gender and cell type.
Interpretation: Gender-dependant slow neurotransmission may convey both genetic and environmental vulnerabilities selectively to SUDs.
Funding: Grants from National Institute on Drug Abuse (NIDA) and National Institute on Alcohol Abuse and Alcoholism (NIAAA) of U.S.A. and National Natural Science Foundation of China (NSFC).
Keywords: Adolescence; Cell type-specific; Environmental risk; Missing heritability; Polysubstance abuse; Slow neurotransmission.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interest.
Figures
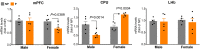


Similar articles
-
Divergent anatomical pattern of D1 and D3 binding and dopamine- and cyclic AMP-regulated phosphoprotein of 32 kDa mRNA expression in the Roman rat strains: Implications for drug addiction.Neuroscience. 2006 Nov 3;142(4):1231-43. doi: 10.1016/j.neuroscience.2006.07.041. Epub 2006 Sep 26. Neuroscience. 2006. PMID: 17008016
-
DARPP-32: from neurotransmission to cancer.Oncotarget. 2016 Apr 5;7(14):17631-40. doi: 10.18632/oncotarget.7268. Oncotarget. 2016. PMID: 26872373 Free PMC article. Review.
-
An association study between PPP1R1B gene and schizophrenia in the Chinese population.Prog Neuropsychopharmacol Biol Psychiatry. 2007 Aug 15;31(6):1303-6. doi: 10.1016/j.pnpbp.2007.05.014. Epub 2007 Jun 7. Prog Neuropsychopharmacol Biol Psychiatry. 2007. PMID: 17618027
-
LncRNA KCNQ1OT1 enhanced the methotrexate resistance of colorectal cancer cells by regulating miR-760/PPP1R1B via the cAMP signalling pathway.J Cell Mol Med. 2019 Jun;23(6):3808-3823. doi: 10.1111/jcmm.14071. Epub 2019 Apr 17. J Cell Mol Med. 2019. PMID: 30997746 Free PMC article.
-
Genetic evidence for role of integration of fast and slow neurotransmission in schizophrenia.Mol Psychiatry. 2017 Jun;22(6):792-801. doi: 10.1038/mp.2017.33. Epub 2017 Mar 28. Mol Psychiatry. 2017. PMID: 28348379 Free PMC article. Review.
Cited by
-
Hypoxic Preconditioned Neural Stem Cell-Derived Extracellular Vesicles Contain Distinct Protein Cargo from Their Normal Counterparts.Curr Issues Mol Biol. 2023 Mar 1;45(3):1982-1997. doi: 10.3390/cimb45030127. Curr Issues Mol Biol. 2023. PMID: 36975497 Free PMC article.
-
Severe COVID-19 in Alzheimer's disease: APOE4's fault again?Alzheimers Res Ther. 2021 Jun 12;13(1):111. doi: 10.1186/s13195-021-00858-9. Alzheimers Res Ther. 2021. PMID: 34118974 Free PMC article.
-
The dopamine transporter gene SLC6A3: multidisease risks.Mol Psychiatry. 2022 Feb;27(2):1031-1046. doi: 10.1038/s41380-021-01341-5. Epub 2021 Oct 14. Mol Psychiatry. 2022. PMID: 34650206 Free PMC article.
-
Back-translating GWAS findings to animal models reveals a role for Hgfac and Slc39a8 in alcohol and nicotine consumption.Sci Rep. 2022 Jun 4;12(1):9336. doi: 10.1038/s41598-022-13283-1. Sci Rep. 2022. PMID: 35661789 Free PMC article.
References
-
- Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–1994. - PMC - PubMed
-
- Manthey J., Shield K.D., Rylett M., Hasan O.S.M., Probst C., Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393(10190):2493–2502. - PubMed
-
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294(5544):1024–1030. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

