Novel Sulfonamide-Based Analogs of Metformin Exert Promising Anti-Coagulant Effects without Compromising Glucose-Lowering Activity
- PMID: 33096688
- PMCID: PMC7589829
- DOI: 10.3390/ph13100323
Novel Sulfonamide-Based Analogs of Metformin Exert Promising Anti-Coagulant Effects without Compromising Glucose-Lowering Activity
Abstract
Metformin, one of the most frequently prescribed oral anti-diabetic drugs, is characterized by multidirectional activity, including lipid lowering, cardio-protective and anti-inflammatory properties. This study presents synthesis and stability studies of 10 novel sulfonamide-based derivatives of metformin with alkyl substituents in the aromatic ring. The potential of the synthesized compounds as glucose-lowering agents and their effects on selected parameters of plasma and vascular hemostasis were examined. Compounds with two or three methyl groups in the aromatic ring (6, 7, 9, 10) significantly increased glucose uptake in human umbilical vein endothelial cells (HUVECs), e.g., 15.8 µmol/L for comp. 6 at 0.3 µmol/mL versus 11.4 ± 0.7 µmol/L for control. Basic coagulation studies showed that all examined compounds inhibit intrinsic coagulation pathway and the process of fibrin polymerization stronger than the parent drug, metformin, which give evidence of their greater anti-coagulant properties. Importantly, synthesized compounds decrease the activity of factor X, a first member of common coagulation pathway, while metformin does not affect coagulation factor X (FX) activity. A multiparametric clot formation and lysis test (CL-test) revealed that the examined compounds significantly prolong the onset of clot formation; however, they do not affect the overall potential of clot formation and fibrinolysis. Erythrotoxicity studies confirmed that none of the synthesized compounds exert an adverse effect on erythrocyte integrity, do not contribute to the massive hemolysis and do not interact strongly with the erythrocyte membrane. In summary, chemical modification of metformin scaffold into benzenesulfonamides containing alkyl substituents leads to the formation of potential dual-action agents with comparable glucose-lowering properties and stronger anti-coagulant activity than the parent drug, metformin.
Keywords: biguanide; coagulation; endothelium; hemostasis; metformin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






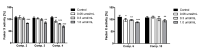


References
-
- Karim S., Shamima Akter F., Jahan Q., Khanom S., Haque A., Yeasmin S., Siddika T., Sinha T. Coagulation Impairment in Type 2 Diabetes Mellitus. J. Bangladesh Soc. Physiol. 2015;10:26–29. doi: 10.3329/jbsp.v10i1.24614. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

