Next-Generation Pertussis Vaccines Based on the Induction of Protective T Cells in the Respiratory Tract
- PMID: 33096737
- PMCID: PMC7711671
- DOI: 10.3390/vaccines8040621
Next-Generation Pertussis Vaccines Based on the Induction of Protective T Cells in the Respiratory Tract
Abstract
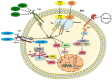
Immunization with current acellular pertussis (aP) vaccines protects against severe pertussis, but immunity wanes rapidly after vaccination and these vaccines do not prevent nasal colonization with Bordetella pertussis. Studies in mouse and baboon models have demonstrated that Th1 and Th17 responses are integral to protective immunity induced by previous infection with B. pertussis and immunization with whole cell pertussis (wP) vaccines. Mucosal Th17 cells, IL-17 and secretory IgA (sIgA) are particularly important in generating sustained sterilizing immunity in the nasal cavity. Current aP vaccines induce potent IgG and Th2-skewed T cell responses but are less effective at generating Th1 and Th17 responses and fail to prime respiratory tissue-resident memory T (TRM) cells, that maintain long-term immunity at mucosal sites. In contrast, a live attenuated pertussis vaccine, pertussis outer membrane vesicle (OMV) vaccines or aP vaccines formulated with novel adjuvants do induce cellular immune responses in the respiratory tract, especially when delivered by the intranasal route. An increased understanding of the mechanisms of sustained protective immunity, especially the role of respiratory TRM cells, will facilitate the development of next generation pertussis vaccines that not only protect against pertussis disease, but prevent nasal colonization and transmission of B. pertussis.
Keywords: Bordetella pertussis; T cells; Th1 cells; Th17 cells; memory T cells; pertussis vaccine.
Conflict of interest statement
Kingston Mills is inventor on a patent application on
Figures

References
-
- Cody C.L., Baraff L.J., Cherry J.D., Marcy S.M., Manclark C.R. Nature and Rates of Adverse Reactions Associated with DTP and DT Immunizations in Infants and Children. Pediatrics. 1981;68:650–660. - PubMed
-
- Decker M.D., Edwards K.M., Steinhoff M.C., Rennels M.B., Pichichero M.E., Englund J.A., Anderson E.L., Deloria M.A., Reed G.F. Comparison of 13 acellular pertussis vaccines: Adverse reactions. Pediatrics. 1995;96:557–566. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

