Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020
- PMID: 33096812
- PMCID: PMC7589810
- DOI: 10.3390/ijms21207794
Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020
Abstract
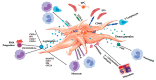
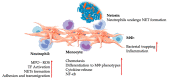
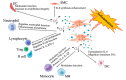
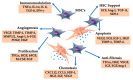
Emerging autologous cellular therapies that utilize platelet-rich plasma (PRP) applications have the potential to play adjunctive roles in a variety of regenerative medicine treatment plans. There is a global unmet need for tissue repair strategies to treat musculoskeletal (MSK) and spinal disorders, osteoarthritis (OA), and patients with chronic complex and recalcitrant wounds. PRP therapy is based on the fact that platelet growth factors (PGFs) support the three phases of wound healing and repair cascade (inflammation, proliferation, remodeling). Many different PRP formulations have been evaluated, originating from human, in vitro, and animal studies. However, recommendations from in vitro and animal research often lead to different clinical outcomes because it is difficult to translate non-clinical study outcomes and methodology recommendations to human clinical treatment protocols. In recent years, progress has been made in understanding PRP technology and the concepts for bioformulation, and new research directives and new indications have been suggested. In this review, we will discuss recent developments regarding PRP preparation and composition regarding platelet dosing, leukocyte activities concerning innate and adaptive immunomodulation, serotonin (5-HT) effects, and pain killing. Furthermore, we discuss PRP mechanisms related to inflammation and angiogenesis in tissue repair and regenerative processes. Lastly, we will review the effect of certain drugs on PRP activity, and the combination of PRP and rehabilitation protocols.
Keywords: analgesic effects; angiogenesis; immunomodulation; inflammation; lymphocytes; monocytes; neutrophils; platelet dosing; platelet-rich plasma; regenerative medicine; rehabilitation; serotonin.
Conflict of interest statement
P.E. is Chief Scientific Officer of EmCyte Corporation and Director Gulf Coast Biologics. All other authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

