Mutation of Arabidopsis Copper-Containing Amine Oxidase Gene AtCuAOδ Alters Polyamines, Reduces Gibberellin Content and Affects Development
- PMID: 33096855
- PMCID: PMC7589035
- DOI: 10.3390/ijms21207789
Mutation of Arabidopsis Copper-Containing Amine Oxidase Gene AtCuAOδ Alters Polyamines, Reduces Gibberellin Content and Affects Development
Abstract
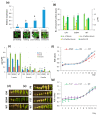
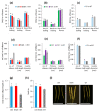
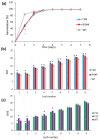
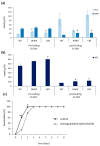
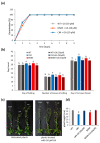
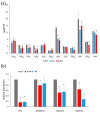
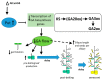
Polyamines (PAs) are essential metabolites in plants performing multiple functions during growth and development. Copper-containing amine oxidases (CuAOs) catalyse the catabolism of PAs and in Arabidopsis thaliana are encoded by a gene family. Two mutants of one gene family member, AtCuAOδ, showed delayed seed germination, leaf emergence, and flowering time. The height of the primary inflorescence shoot was reduced, and developmental leaf senescence was delayed. Siliques were significantly longer in mutant lines and contained more seeds. The phenotype of AtCuAOδ over-expressors was less affected. Before flowering, there was a significant increase in putrescine in AtCuAOδ mutant leaves compared to wild type (WT), while after flowering both spermidine and spermine concentrations were significantly higher than in WT leaves. The expression of GA (gibberellic acid) biosynthetic genes was repressed and the content of GA1, GA7, GA8, GA9, and GA20 was reduced in the mutants. The inhibitor of copper-containing amine oxidases, aminoguanidine hydrochloride, mimicked the effect of AtCuAOδ mutation on WT seed germination. Delayed germination, reduced shoot height, and delayed flowering in the mutants were rescued by GA3 treatment. These data strongly suggest AtCuAOδ is an important gene regulating PA homeostasis, and that a perturbation of PAs affects plant development through a reduction in GA biosynthesis.
Keywords: copper-containing amine oxidases; flowering; gibberellic acid; polyamines; putrescine; senescence.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Davies P.J. The plant hormones: Their nature, occurrence, and functions. In: Davies P.J., editor. Plant Hormones: Biosynthesis, Signal Transduction and Action. 3rd ed. Springer; Dordrecht, The Netherlands: 2010. pp. 1–15.
-
- Michael A.J. Biosynthesis of polyamines in eukaryotes, archaea, and bacteria. In: Kusano T., Suzuki H., editors. Polyamines. Springer; Tokyo, Japan: 2015. pp. 3–14.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

