Intranasal Administration of Mesenchymal Stem Cell Secretome Reduces Hippocampal Oxidative Stress, Neuroinflammation and Cell Death, Improving the Behavioral Outcome Following Perinatal Asphyxia
- PMID: 33096871
- PMCID: PMC7589575
- DOI: 10.3390/ijms21207800
Intranasal Administration of Mesenchymal Stem Cell Secretome Reduces Hippocampal Oxidative Stress, Neuroinflammation and Cell Death, Improving the Behavioral Outcome Following Perinatal Asphyxia
Abstract
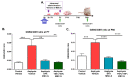
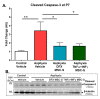
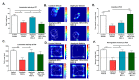
Perinatal Asphyxia (PA) is a leading cause of motor and neuropsychiatric disability associated with sustained oxidative stress, neuroinflammation, and cell death, affecting brain development. Based on a rat model of global PA, we investigated the neuroprotective effect of intranasally administered secretome, derived from human adipose mesenchymal stem cells (MSC-S), preconditioned with either deferoxamine (an hypoxia-mimetic) or TNF-α+IFN-γ (pro-inflammatory cytokines). PA was generated by immersing fetus-containing uterine horns in a water bath at 37 °C for 21 min. Thereafter, 16 μL of MSC-S (containing 6 μg of protein derived from 2 × 105 preconditioned-MSC), or vehicle, were intranasally administered 2 h after birth to asphyxia-exposed and control rats, evaluated at postnatal day (P) 7. Alternatively, pups received a dose of either preconditioned MSC-S or vehicle, both at 2 h and P7, and were evaluated at P14, P30, and P60. The preconditioned MSC-S treatment (i) reversed asphyxia-induced oxidative stress in the hippocampus (oxidized/reduced glutathione); (ii) increased antioxidative Nuclear Erythroid 2-Related Factor 2 (NRF2) translocation; (iii) increased NQO1 antioxidant protein; (iv) reduced neuroinflammation (decreasing nuclearNF-κB/p65 levels and microglial reactivity); (v) decreased cleaved-caspase-3 cell-death; (vi) improved righting reflex, negative geotaxis, cliff aversion, locomotor activity, anxiety, motor coordination, and recognition memory. Overall, the study demonstrates that intranasal administration of preconditioned MSC-S is a novel therapeutic strategy that prevents the long-term effects of perinatal asphyxia.
Keywords: Neonatal hypoxia; behavioral development; cell death; hippocampus; intranasal administration; memory; mesenchymal stem cell secretome (MSC-S); neuroinflammation; neuroprotection; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Antonucci R., Porcella A., Pilloni M.D. Perinatal asphyxia in the term newborn. J. Pediatr. Neonatal Individ. Med. 2014;3:e030269. doi: 10.7363/030269. - DOI
-
- Morales P., Fiedler J.L., Andrés S., Berrios C., Huaiquín P., Bustamante D., Cardenas S., Parra E., Herrera-Marschitz M. Plasticity of hippocampus following perinatal asphyxia: Effects on postnatal apoptosis and neurogenesis. J. Neurosci. Res. 2008;86:2650–2662. doi: 10.1002/jnr.21715. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

