Methanosarcina acetivorans contains a functional ISC system for iron-sulfur cluster biogenesis
- PMID: 33096982
- PMCID: PMC7585200
- DOI: 10.1186/s12866-020-02014-z
Methanosarcina acetivorans contains a functional ISC system for iron-sulfur cluster biogenesis
Abstract
Background: The production of methane by methanogens is dependent on numerous iron-sulfur (Fe-S) cluster proteins; yet, the machinery involved in Fe-S cluster biogenesis in methanogens remains largely unknown. Methanogen genomes encode uncharacterized homologs of the core components of the ISC (IscS and IscU) and SUF (SufBC) Fe-S cluster biogenesis systems found in bacteria and eukaryotes. Methanosarcina acetivorans contains three iscSU and two sufCB gene clusters. Here, we report genetic and biochemical characterization of M. acetivorans iscSU2.
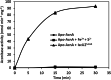
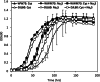
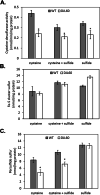
Results: Purified IscS2 exhibited pyridoxal 5'- phosphate-dependent release of sulfur from L-cysteine. Incubation of purified IscU2 with IscS2, cysteine, and iron (Fe2+) resulted in the formation of [4Fe-4S] clusters in IscU2. IscU2 transferred a [4Fe-4S] cluster to purified M. acetivorans apo-aconitase. IscU2 also restored the aconitase activity in air-exposed M. acetivorans cell lysate. These biochemical results demonstrate that IscS2 is a cysteine desulfurase and that IscU2 is a Fe-S cluster scaffold. M. acetivorans strain DJL60 deleted of iscSU2 was generated to ascertain the in vivo importance of IscSU2. Strain DJL60 had Fe-S cluster content and growth similar to the parent strain but lower cysteine desulfurase activity. Strain DJL60 also had lower intracellular persulfide content compared to the parent strain when cysteine was an exogenous sulfur source, linking IscSU2 to sulfur metabolism.
Conclusions: This study establishes that M. acetivorans contains functional IscS and IscU, the core components of the ISC Fe-S cluster biogenesis system and provides the first evidence that ISC operates in methanogens.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

