The histopathology of SPINK1-associated chronic pancreatitis
- PMID: 33097431
- PMCID: PMC7704661
- DOI: 10.1016/j.pan.2020.10.030
The histopathology of SPINK1-associated chronic pancreatitis
Abstract
Background: The identification of genetic risk factors for chronic pancreatitis, such as PRSS1, CFTR and SPINK1, provides the opportunity to define key pathologic hallmarks and etiologic-specific changes. For example, pancreata from PRSS1 and CFTR patients exhibit progressive lipomatous atrophy without significant fibrosis. Considering the pathology of SPINK1-associated pancreatitis is ill-defined, we examined the pancreata of SPINK1 patients with chronic pancreatitis.
Methods: Histologic sections after total pancreatectomy with islet autotransplantation and associated clinicopathologic data were collected from 28 patients with SPINK1 germline alterations. Clinical findings, germline data, anatomic anomalies and pathologic findings were descriptively evaluated.
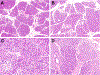
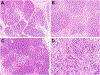
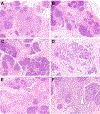
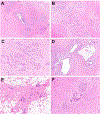
Results: Patients ranged in age from 5 to 48 years (median, 21.6 years) with abdominal pain between 2 and 25 years (median, 5.8 years). Most patients were SPINK1 heterozygous and 14 (50%) had co-occurring CFTR (n = 12) and CTRC (n = 2) mutations. Other pancreatitis risk factors included anatomic anomalies (n = 9) and tobacco use (n = 1). Overall, 24 (86%) patients had additional pancreatitis-associated germline alterations, SPINK1 homozygosity, anatomic anomalies or environmental factors. Examination of pancreata revealed a sequential pattern of exocrine parenchymal loss and replacement by prominent fibrosis, dependent on the duration of abdominal pain. No malignancies were identified, but low-grade pancreatic intraepithelial neoplasia was present for 2 cases.
Conclusions: Within this descriptive study, SPINK1-associated pancreatitis is characterized by parenchymal fibrosis and suggests divergent pathophysiologic mechanisms from PRSS1 and CFTR-associated pancreatitis. Moreover, SPINK1 patients frequently had additional etiologic factors that did not impact the development of pancreatic fibrosis and may implicate SPINK1 as a disease modifier gene.
Keywords: Alcohol; Fibrosis; Genetics; Pancreatitis; Pathology.
Copyright © 2020 IAP and EPC. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts of interest to declare.
Figures
References
-
- Kleeff J, Whitcomb DC, Shimosegawa T, et al. Chronic pancreatitis. Nat Rev Dis Primers 2017;3:17060. - PubMed
-
- Sossenheimer MJ, Aston CE, Preston RA, et al. Clinical characteristics of hereditary pancreatitis in a large family, based on high-risk haplotype. The Midwest Multicenter Pancreatic Study Group (MMPSG). Am J Gastroenterol 1997;92:1113–6. - PubMed
-
- Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2004;2:252–61. - PubMed
-
- de las Heras-Castano G, Castro-Senosiain B, Fontalba A, et al. Hereditary pancreatitis: clinical features and inheritance characteristics of the R122C mutation in the cationic trypsinogen gene (PRSS1) in six Spanish families. JOP 2009;10:249–55. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

