Hexachlorobenzene Monooxygenase Substrate Selectivity and Catalysis: Structural and Biochemical Insights
- PMID: 33097503
- PMCID: PMC7755259
- DOI: 10.1128/AEM.01965-20
Hexachlorobenzene Monooxygenase Substrate Selectivity and Catalysis: Structural and Biochemical Insights
Abstract
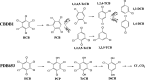
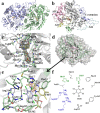
Hexachlorobenzene (HCB), as one of the persistent organic pollutants (POPs) and a possible human carcinogen, is especially resistant to biodegradation. In this study, HcbA1A3, a distinct flavin-N5-peroxide-utilizing enzyme and the sole known naturally occurring aerobic HCB dechlorinase, was biochemically characterized. Its apparent preference for HCB in binding affinity revealed that HcbA1 could oxidize only HCB rather than less-chlorinated benzenes such as pentachlorobenzene and tetrachlorobenzenes. In addition, the crystal structure of HcbA1 and its complex with flavin mononucleotide (FMN) were resolved, revealing HcbA1 to be a new member of the bacterial luciferase-like family. A much smaller substrate-binding pocket of HcbA1 than is seen with its close homologues suggests a requirement of limited space for catalysis. In the active center, Tyr362 and Asp315 are necessary in maintaining the normal conformation of HcbA1, while Arg311, Arg314, Phe10, Val59, and Met12 are pivotal for the substrate affinity. They are supposed to place HCB at a productive orientation through multiple interactions. His17, with its close contact with the site of oxidation of HCB, probably fixes the target chlorine atom and stabilizes reaction intermediates. The enzymatic characteristics and crystal structures reported here provide new insights into the substrate specificity and catalytic mechanism of HcbA1, which paves the way for its rational engineering and application in the bioremediation of HCB-polluted environments.IMPORTANCE As an endocrine disrupter and possible carcinogen to human beings, hexachlorobenzene (HCB) is especially resistant to biodegradation, largely due to difficulty in its dechlorination. The lack of knowledge of HCB dechlorinases limits their application in bioremediation. Recently, an HCB monooxygenase, HcbA1A3, representing the only naturally occurring aerobic HCB dechlorinase known so far, was reported. Here, we report its biochemical and structural characterization, providing new insights into its substrate selectivity and catalytic mechanism. This research also increases our understanding of HCB dechlorinases and flavin-N5-peroxide-utilizing enzymes.
Keywords: biodegradation; catalytic mechanism; crystal structure; dechlorinase; flavin-N5-peroxide; hexachlorobenzene; monooxygenase.
Copyright © 2020 American Society for Microbiology.
Figures




Similar articles
-
Biochemical characterization of NADH:FMN oxidoreductase HcbA3 from Nocardioides sp. PD653 in catalyzing aerobic HCB dechlorination.J Pestic Sci. 2020 Aug 20;45(3):125-131. doi: 10.1584/jpestics.D20-23. J Pestic Sci. 2020. PMID: 32913414 Free PMC article.
-
Mechanisms of aerobic dechlorination of hexachlorobenzene and pentachlorophenol by Nocardioides sp. PD653.J Pestic Sci. 2021 Nov 20;46(4):373-381. doi: 10.1584/jpestics.J21-04. J Pestic Sci. 2021. PMID: 34908898 Free PMC article.
-
Identification of the hcb Gene Operon Involved in Catalyzing Aerobic Hexachlorobenzene Dechlorination in Nocardioides sp. Strain PD653.Appl Environ Microbiol. 2017 Sep 15;83(19):e00824-17. doi: 10.1128/AEM.00824-17. Print 2017 Oct 1. Appl Environ Microbiol. 2017. PMID: 28733287 Free PMC article.
-
Engineering substrate recognition in catalysis by cytochrome P450cam.Biochem Soc Trans. 2003 Jun;31(Pt 3):558-62. doi: 10.1042/bst0310558. Biochem Soc Trans. 2003. PMID: 12773156 Review.
-
Dynamics involved in catalysis by single-component and two-component flavin-dependent aromatic hydroxylases.Biochem Biophys Res Commun. 2005 Dec 9;338(1):590-8. doi: 10.1016/j.bbrc.2005.09.081. Epub 2005 Sep 26. Biochem Biophys Res Commun. 2005. PMID: 16236251 Review.
Cited by
-
Critical Role of Monooxygenase in Biodegradation of 2,4,6-Trinitrotoluene by Buttiauxella sp. S19-1.Molecules. 2023 Feb 19;28(4):1969. doi: 10.3390/molecules28041969. Molecules. 2023. PMID: 36838956 Free PMC article.
-
Structural, biophysical, and biochemical insights into C-S bond cleavage by dimethylsulfone monooxygenase.Proc Natl Acad Sci U S A. 2024 Nov 19;121(47):e2401858121. doi: 10.1073/pnas.2401858121. Epub 2024 Nov 12. Proc Natl Acad Sci U S A. 2024. PMID: 39531498 Free PMC article.
-
A Multicomponent THF Hydroxylase Initiates Tetrahydrofuran Degradation in Cupriavidus metallidurans ZM02.Appl Environ Microbiol. 2022 Mar 22;88(6):e0188021. doi: 10.1128/AEM.01880-21. Epub 2022 Mar 22. Appl Environ Microbiol. 2022. PMID: 35108100 Free PMC article.
-
Structural and Functional Characterization of a Novel Class A Flavin Monooxygenase from Bacillus niacini.Biochemistry. 2024 Oct 1;63(19):2506-2516. doi: 10.1021/acs.biochem.4c00306. Epub 2024 Sep 12. Biochemistry. 2024. PMID: 39265075
-
Ligand bound structure of a 6-hydroxynicotinic acid 3-monooxygenase provides mechanistic insights.Arch Biochem Biophys. 2024 Feb;752:109859. doi: 10.1016/j.abb.2023.109859. Epub 2023 Dec 16. Arch Biochem Biophys. 2024. PMID: 38104959 Free PMC article.
References
-
- Antunes P, Viana P, Vinhas T, Rivera J, Gaspar EM. 2012. Emission profiles of polychlorinated dibenzodioxins, polychlorinated dibenzofurans (PCDD/Fs), dioxin-like PCBs and hexachlorobenzene (HCB) from secondary metallurgy industries in Portugal. Chemosphere 88:1332–1339. doi:10.1016/j.chemosphere.2012.05.032. - DOI - PubMed
-
- Ahmed G, Anawar HM, Takuwa DT, Chibua IT, Singh GS, Sichilongo K. 2015. Environmental assessment of fate, transport and persistent behavior of dichlorodiphenyltrichloroethanes and hexachlorocyclohexanes in land and water ecosystems. Int J Environ Sci Technol 12:2741–2756. doi:10.1007/s13762-015-0792-3. - DOI
-
- Robles-Molina J, Gilbert-López B, García-Reyes JF, Molina-Díaz A. 2014. Monitoring of selected priority and emerging contaminants in the Guadalquivir River and other related surface waters in the province of Jaén, South East Spain. Sci Total Environ 479–480:247–257. doi:10.1016/j.scitotenv.2014.01.121. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

