Soil Characteristics Constrain the Response of Microbial Communities and Associated Hydrocarbon Degradation Genes during Phytoremediation
- PMID: 33097512
- PMCID: PMC7783334
- DOI: 10.1128/AEM.02170-20
Soil Characteristics Constrain the Response of Microbial Communities and Associated Hydrocarbon Degradation Genes during Phytoremediation
Abstract
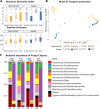
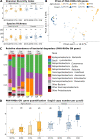
Rhizodegradation is a promising cleanup technology where microorganisms degrade soil contaminants in the rhizosphere. A symbiotic relationship is expected to occur between plant roots and soil microorganisms in contaminated soils that enhances natural microbial degradation. However, little is known about how different initial microbiotas influence the rhizodegradation outcome. Recent studies have hinted that soil initial diversity has a determining effect on the outcome of contaminant degradation. To test this, we either planted (P) or not (NP) balsam poplars (Populus balsamifera) in two soils of contrasting diversity (agricultural and forest) that were contaminated or not with 50 mg kg-1 of phenanthrene (PHE). The DNA from the rhizosphere of the P and the bulk soil of the NP pots was extracted and the bacterial genes encoding the 16S rRNA, the PAH ring-hydroxylating dioxygenase alpha subunits (PAH-RHDα) of Gram-positive and Gram-negative bacteria, and the fungal ITS region were sequenced to characterize the microbial communities. The abundances of the PAH-RHDα genes were quantified by real-time quantitative PCR. Plant presence had a significant effect on PHE degradation only in the forest soil, whereas both NP and P agricultural soils degraded the same amount of PHE. Fungal communities were mainly affected by plant presence, whereas bacterial communities were principally affected by the soil type, and upon contamination the dominant PAH-degrading community was similarly constrained by soil type. Our results highlight the crucial importance of soil microbial and physicochemical characteristics in the outcome of rhizoremediation.IMPORTANCE Polycyclic aromatic hydrocarbons (PAH) are a group of organic contaminants that pose a risk to ecosystems' health. Phytoremediation is a promising biotechnology with the potential to restore PAH-contaminated soils. However, some limitations prevent it from becoming the remediation technology of reference, despite being environmentally friendlier than mainstream physicochemical alternatives. Recent reports suggest that the original soil microbial diversity is the key to harnessing the potential of phytoremediation. Therefore, this study focused on determining the effect of two different soil types in the fate of phenanthrene (a polycyclic aromatic hydrocarbon) under balsam poplar remediation. Poplar increased the degradation of phenanthrene in forest, but not in agricultural soil. The fungi were affected by poplars, whereas total bacteria and specific PAH-degrading bacteria were constrained by soil type, leading to different degradation patterns between soils. These results highlight the importance of performing preliminary microbiological studies of contaminated soils to determine whether plant presence could improve remediation rates or not.
Keywords: phenanthrene; phytoremediation; polycyclic aromatic hydrocarbons; poplar; rhizosphere-inhabiting microbes; soil contamination; soil diversity.
Copyright © 2021 American Society for Microbiology.
Figures



Similar articles
-
Impact of clay mineral, wood sawdust or root organic matter on the bacterial and fungal community structures in two aged PAH-contaminated soils.Environ Sci Pollut Res Int. 2015 Sep;22(18):13724-38. doi: 10.1007/s11356-015-4117-3. Epub 2015 Jan 25. Environ Sci Pollut Res Int. 2015. PMID: 25616383
-
Phenanthrene contamination and ploidy level affect the rhizosphere bacterial communities of Spartina spp.FEMS Microbiol Ecol. 2020 Oct 2;96(10):fiaa156. doi: 10.1093/femsec/fiaa156. FEMS Microbiol Ecol. 2020. PMID: 32821911
-
Differential degradation of polycyclic aromatic hydrocarbon mixtures by indigenous microbial assemblages in soil.Lett Appl Microbiol. 2015 Aug;61(2):199-207. doi: 10.1111/lam.12446. Epub 2015 Jun 24. Lett Appl Microbiol. 2015. PMID: 26031321
-
Prospects for arbuscular mycorrhizal fungi (AMF) to assist in phytoremediation of soil hydrocarbon contaminants.Chemosphere. 2016 Nov;162:105-16. doi: 10.1016/j.chemosphere.2016.07.071. Epub 2016 Jul 31. Chemosphere. 2016. PMID: 27487095 Review.
-
The rhizosphere microbiome: Significance in rhizoremediation of polyaromatic hydrocarbon contaminated soil.J Environ Manage. 2018 Jul 1;217:858-870. doi: 10.1016/j.jenvman.2018.04.022. Epub 2018 Apr 24. J Environ Manage. 2018. PMID: 29660711 Review.
Cited by
-
Microbiological Study in Petrol-Spiked Soil.Molecules. 2021 May 1;26(9):2664. doi: 10.3390/molecules26092664. Molecules. 2021. PMID: 34062889 Free PMC article.
-
DNA stable isotope probing on soil treated by plant biostimulation and flooding revealed the bacterial communities involved in PCB degradation.Sci Rep. 2022 Nov 10;12(1):19232. doi: 10.1038/s41598-022-23728-2. Sci Rep. 2022. PMID: 36357494 Free PMC article.
-
Soil initial bacterial diversity and nutrient availability determine the rate of xenobiotic biodegradation.Microb Biotechnol. 2022 Jan;15(1):318-336. doi: 10.1111/1751-7915.13946. Epub 2021 Oct 24. Microb Biotechnol. 2022. PMID: 34689422 Free PMC article.
-
Soil fauna-microbial interactions shifts fungal and bacterial communities under a contamination disturbance.PLoS One. 2023 Oct 25;18(10):e0292227. doi: 10.1371/journal.pone.0292227. eCollection 2023. PLoS One. 2023. PMID: 37878639 Free PMC article.
-
Advancement of Metatranscriptomics towards Productive Agriculture and Sustainable Environment: A Review.Int J Mol Sci. 2022 Mar 29;23(7):3737. doi: 10.3390/ijms23073737. Int J Mol Sci. 2022. PMID: 35409097 Free PMC article. Review.
References
-
- Jonsson FA. 2012. The industrial revolution in the Anthropocene. J Mod Hist 84:679–696. doi:10.1086/666049. - DOI
-
- Jones KC, Stratford JA, Waterhouse KS, Furlong ET, Giger W, Hites RA, Schaffner C, Johnston AE. 1989. Increases in the polynuclear aromatic hydrocarbon content of an agricultural soil over the last century. Environ Sci Technol 23:95–101. doi:10.1021/es00178a012. - DOI
-
- Ravindra K, Sokhi R, Van Grieken R. 2008. Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ 42:2895–2921. doi:10.1016/j.atmosenv.2007.12.010. - DOI
-
- Menzie CA, Potocki BB, Joseph S. 1992. Exposure to carcinogenic PAHs in the environment. Environ Sci Technol 26:1278–1284. doi:10.1021/es00031a002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

